
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
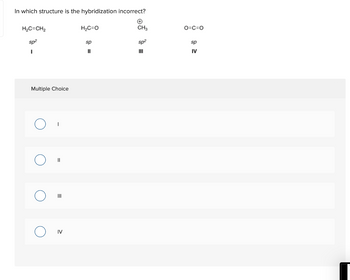
Transcribed Image Text:In which structure is the hybridization incorrect?
H₂C=CH₂
sp²
I
Multiple Choice
I
||
III
IV
H₂C=O
sp
11
CH3
sp²
III
O=C=O
sp
IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5arrow_forwardGive approximate values for the indicated bond angles in the molecule shown. 5,6 = Express the bonding angles in degrees to four significant digits separated by a comma. VE ΑΣΦ H 5 T H N-Q-H ? 6arrow_forwardConsider the two structures shown below: + C6 Which of the following statements is incorrect? O c is bonded to only one hydrogen. o cD is sp²-hybridized. C has a nonbonded lone pair of electrons. C is bonded to only one hydrogen. The octet rule is satisfied for CD.arrow_forward
- What is the hybridization of Kr in KrF2? (Kr in middle) Give all atoms zero formal charge. O sp O sp O sp3 O sp³d Pęds Oarrow_forward#23arrow_forwardComplete the following table: Lewis structure atoms: bonds between orbitals making each total bond order hybridization atoms: type E valence bond: between two atoms |Н: 1s C and H: o sp?(C) + 1s(H) C and H: 1 C: sp? Cl: sp3 0: sp? :0: || C and Cl: o sp?(C) + sp°(CI) C and Cl: 1 = sp?(C) + sp²(0) = p(C) + p(0) C and O: o C and O: 2 CI C and O: t C: C and C: C and C: C and C: C: H-C=C-H H: C and H: C and C: H: C and H: N: N and I: N and I: N= I: N and I: C: C and Cl: C and Cl: :Cl Cl: C and Cl: C and Cl: Cl: C and Cl: -c=cl: C and Cl: :Z: :U:arrow_forward
- I honestly have no clue what to doarrow_forwardUse the blank MO diagram (below) to aid in answering the question. Calculate the bond order for NO. + E — Пр - Op - Пр * — Пр — Op — Пр Os Os estion 19 15 DEC 10arrow_forwardWhat hybridization best describes the circled atom? 1-N I O sp O sp² O sp²-sp³ O sp³ N N -Narrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY