
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
In titration we are interested in the equivalence point. Chemically this is when enough of the base has been added to completely neutralize the acid. Graphically this is when the reaction rate begins to decrease. How do we determine the equivalence point from P? Next, how do we determine the equivalence point from P'? What does the data suggest? Estimate the equivalence point with these two approaches using your data and graph.
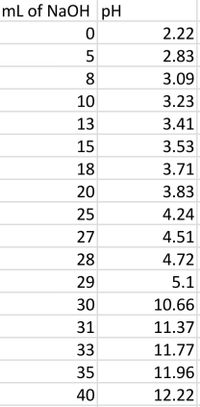
Transcribed Image Text:This dataset shows the pH changes in a solution as sodium hydroxide (NaOH) is added. The table consists of two columns: "mL of NaOH" and "pH."
### Data Table
- **mL of NaOH**
- 0
- 5
- 8
- 10
- 13
- 15
- 18
- 20
- 25
- 27
- 28
- 29
- 30
- 31
- 33
- 35
- 40
- **pH**
- 2.22
- 2.83
- 3.09
- 3.23
- 3.41
- 3.53
- 3.71
- 3.83
- 4.24
- 4.51
- 4.72
- 5.1
- 10.66
- 11.37
- 11.77
- 11.96
- 12.22
### Analysis
- **Initial Stage (0-20 mL):** The pH gradually increases from 2.22 to 3.83, indicating a weak acidic solution being neutralized.
- **Buffer Region (20-29 mL):** The pH continues to rise slowly, from 3.83 to 5.1, suggesting the presence of a buffering system.
- **Equivalence Point and Beyond (30-40 mL):** A rapid increase in pH occurs, reaching alkalinity with values from 10.66 to 12.22. This suggests the neutralization of acidic components and the dominance of NaOH.
This pattern is typical in titration, where a solution transitions from acidic to basic as a strong base is added. The dramatic rise in pH around 30 mL indicates the point where the acid is fully neutralized.

Transcribed Image Text:Titration is a method of chemical analysis in which a reactive substance is slowly added to another substance, and some property of the combined substance is measured. This procedure is taught in CHM 116, General Chemistry II.
We will be looking at an example of acid-base titration. Specifically, 25 mL of an unknown monoprotic weak acid is titrated against 0.105M NaOH (which is a strong base). This means we are adding the base to the acid using a burette in a slow and precisely controlled manner. We measure the pH after each addition. (A pH of less than 7 is an acid, and a pH of more than 7 is a base.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please answer questions 3a&b based on the information in question 2.arrow_forwardI need help with the first derivative plot and the second derivative plot? Can you plot the data tables with x and y values for both derivative plots, then I can graph it myarrow_forwardThe preparation of an aqueous solution is described in the table below. For this solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCH,CO₂ is a weak acid. 0.3 mol of NaOH is added to 1.0 L of a solution that is 0.7M in both HCH,CO, and NaCH, CO₂. acids: O bases: O Oother: D 0.0arrow_forward
- The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. 0.61 mol of HCl is added to 1.0 L of a 1.3 MNH₂ solution. 0.1 mol of HNO3 is added to 1.0 L of a solution that is 0.4M in both NH3 and NH₂Cl. acids: bases: other: acids: bases: other: 7⁰ X S ...arrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCH₂CO, is a weak acid. 2.3 mol of KOH is added to 1.0 L of a 1.2MHCH, CO₂ solution. 0.42 mol of KOH is added to 1.0 L of a solution that is 1.3M in both HCH₂CO₂ and NaCH3CO₂ acids: 0 Obases: Oother: O O acids: 0 Obases: 0 0 other: 10 X 2 00 9 0.0. Sarrow_forwardConsider a solution containing reactants and only trace amounts of products. The reaction proceeds over a very short period of time, and produces a small amount of product. Which of the following is true?a. The Gibbs energy of the solution is higher than the Gibbs energy of the solution at equilibriumb. The chemical potential of the products is higher than the chemical potential of the reactantsc. The Gibbs energy of the reaction is greater than 0d. None of the abovearrow_forward
- The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCH3CO₂ is a weak acid. 0.7 mol of NaOH is added to 1.0 L of a 0.7 M HCH3CO₂ solution. 0.10 mol of HNO3 is added to 1.0 L of a solution that is 1.1M in both HCH₂CO₂ and KCH3CO2. acids: 0 bases: other: acids: 0 bases: other: × 0,0,... Śarrow_forwardThe preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that NH3 is a weak base. 0.70 mol of HI is added to 1.0 L of a 1.5M NH3 solution. 0.1 mol of HNO3 is added to 1.0 L of a solution that is 0.4M in both NH3 and NH Br. 4 acids: bases: other: acids: bases: other: X 0,0,... Śarrow_forwardSolubility Calculations Given that the average concentration of calcium ion in natural waters is 3.8x10-4 M, and that the solubility product Ksp for CaF₂ is 4.0x10-11, calculate the maximum concentration of fluoride ion that can be dissolved before precipitation begins.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY