
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
- In this lab you used acid/base extraction to remove acidic impurities, how could basic impurities be removed through extraction? Imagine the two compounds below are both dissolved in ethyl acetate (a standard organic solvent for extractions), create a diagram/flow chart that outlines steps that could be taken to separate them through acid/base extraction and recover each compound in its neutral Be sure to track when each compound is in the organic or aqueous layer.
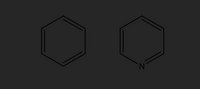
Transcribed Image Text:The image displays two chemical structures, depicted as hexagonal rings:
1. **Left Structure**:
- This is a benzene ring, consisting of six carbon atoms.
- The hexagon features alternating double and single bonds, indicating aromaticity.
2. **Right Structure**:
- This is a pyridine ring, similar in shape to benzene but with one nitrogen atom replacing one of the carbon atoms.
- Like benzene, it retains the alternating double and single bonds, also indicating aromaticity.
Both structures are fundamental in organic chemistry, illustrating the concept of aromatic compounds. Benzene is a simple aromatic hydrocarbon, while pyridine is a basic heterocyclic compound featuring nitrogen.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 1 You are employed as a co-op student at the Drug and Alcohol Testing Association of Canada (DATAC) developing analytical tests for sports doping agents. You are asked to prepare a procedure for the extraction of methylphenidate, the active compound in Ritalin, from urine samples (consider them as simple aqueous layers, you do not need to consider other components!). The goal of the procedure is to extract the methylphenidate into an organic layer which will then be evaporated and the residue will be tested for the drug. You find that methylphenidate is highly soluble in 2-methyltetrahydrofuran, a bio-renewable solvent. Draw the structure of 2-methyltetrahydrofuran and give two reasons why it is a good solvent choice for liquid-liquid extraction. (Please find the image attached below to help you with this question) Question 2 (cont. from scenario 1). Your colleague is helping you develop the urine test. They suggest that the urine should be adjusted to a pH above 7 before…arrow_forwardIn this lab you used acid/base extraction to remove acidic impurities, how could basic impurities be removed through extraction? Imagine the two compounds below are both dissolved in ethyl acetate (a standard organic solvent for extractions), create a diagram/flow chart that outlines steps that could be taken to separate them through acid/base extraction and recover each compound in its neutral Be sure to track when each compound is in the organic or aqueous layer.arrow_forwardA student use the same protocol as in the lab manual to extract 4 chloroanilline, benzoic acid and naphthalene. Below are the steps he took Step 1 - Dissolve all three in 15 ml ether and place in separatory funnel Step 2- Add 15 ml of hydrochloric acid and mix well Step 3 - Isolate the aqueous layer and add sodium hydroxide which resulted in a precipitate Step 4- Add 15 ml of sodium carbonate to the ether layer and mix Step 5- Isolate the aqueous layer and add hydrochloric acid, which resulted in a precipitate. Step 6- dry the ether layer and collect the solid that is leftarrow_forward
- 4. Below is an acid-base extraction flow chart. The four compounds seen in the first box was mixed and dissolved in dichloromethane. Complete the flow chart by drawing (bond-line structure) the organic compound that is found in each layer upon each addition. Draw in any relevant charges. OH CN 10% HCI ORGANIC AQUEOUS 10% NaHCO3 AQUEOUS ORGANIC 10% КОН AQUEOUS ORGANICarrow_forwardPlease answer the question at the bottom, Thanks. LAB NOTES FOR PREPARATION OF BUTYL MAGNESIUM BROMIDEAND ITS SUBSEQUENT CONVERSION TO AN ALCOHOL To the reaction flask were added 2.5967 gram of magnesium turnings, about 20 mlof diglyme, and a solution of 13.5942 gram of butyl bromide in about 25 mldiglyme.The procedure as per the lab manual was initiated. After the required heating timewas completed the reaction mixture was allowed to cool to room temperature.Then a solution of 6.1123 gram of acetone in about 15 ml of diglyme was addeddropwise as per the lab manual.The rest of the lab manual procedure was completed, ultimately affording 2.5532gram of an oily, clear and slightly yellow liquid, presumed to be 2-methyl-2-hexanol. Boiling point as measured by distillation was 138 – 141 deg C.About 0.8 ml of the presumed 2-methyl-2-hexanol was placed in an NMR tube towhich was added 2 drops of TMS. The tube was capped, then inverted and righted20 times to thoroughly mix the product and…arrow_forwardUsing the solubility information from your table of constants, make a flow chart, showing the procedure you would use to separate 4- chloroaniline from 1,4-dibromobenzene. Indicate what compound is in each solvent by writing the structures at the appropriate locations in the flow chart. please answer the question, and draw the chart.arrow_forward
- If 80% of a desired compound is extracted into the organic phase in each step (leaving 20% in the aqueous layer), how many extractions are necessary to ensure that at least 99% of the compound has been recovered in the organic phase? 1 extraction 2 extractions 3 extractions 4 extractionsarrow_forwardA mixture of the two compounds shown below is dissolved in ether. Using the information given in the experiment, select all the correct statements regarding the extraction and isolation of the two compounds. محمد age X Y A. X can be extracted from the organic layer using hydrochloric acid. OB. Y can precipitate if hydrochloric acid is added to the salt of Y in the aqueous layer. OC. Y can be extracted from the organic layer using hydrochloric acid. D. X can precipitate if hydrochloric acid is added to the salt of X in the aqueous layerarrow_forwardSelect all that apply. If dichloromethane (CH2Cl2), benzoic acid, and excess aqueous NaOH are mixed in a separatory funnel, what constituent(s) will primarily be in the organic phase? Select all that apply OGMU 2020 1.Benzoic acid O2.Sodium benzoate 3. Sodium hydoxide 4. None of these O5.CH2Cl2 OURST ION 1Earrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY