
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:23
eBook
Print
References
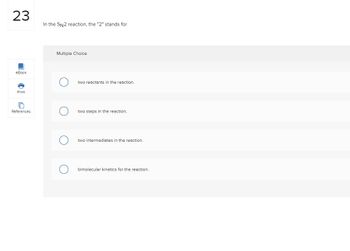
In the SN2 reaction, the "2" stands for
Multiple Choice
two reactants in the reaction.
two steps in the reaction.
two intermediates in the reaction.
bimolecular kinetics for the reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following initial rate data was collected for the reaction: Experi ment 1 2 HI (g) + C₂H5l (g) --> C₂H6 (g) + 1₂ (g) 3 [HI] 0.015 M 0.030 M 0.030 M [C₂H51] 0.900 M 0.900 M 0.450 M Initial Rate 4.01 x 10 5 M/S 8.04 x 10 5 M/s 3.99 x 10 5 M/Sarrow_forwardWhich one of the following factors does not affect the specific rate constant of a chemical reaction? A. Arrhenius probability factor B. Temperature C. Activation barrier D. The presence of a catalyst E. Change in the Gibbs free energy for the reactionarrow_forwardChemistry: Fundamentals and Principles Davidson presented by Macmillan Le Reaction A has a high activation energy, whereas reacton B has a low activation energy. Which of the statements about reaction A and reaction B are true? Reaction A is more likely to occur at all than reaction B. Reaction B is likely to occur at a faster rate than reaction A. Reaction B is more likely to occur at all than reaction A. Reaction A is likely to occur at a faster rate than reaction B. O O Oarrow_forward
- 10.a. A student performed the following kinetic isotope effect experiment. • The reaction below took 15 min to achieve completion. H3C H3C HI Br D3C • The reaction below (note deuterium replacement) took 60 min to achieve completion. CD3 D3C D H Br This group is isopropyl, going backwards into paper NaOEt EtOH -CD3 NaOEt EtOH D Draw the correct mechanism(s) and product(s) for the top reaction with H-labeled reactant. Show stereochemistry clearly.arrow_forward3. Draw a reaction energy diagram, and label the product (P), reactant (R), transition states (TS1, TS2, etc.), intermediates (INT1, INT2, etc.) and activation energies (AG‡₁, AG 2, etc.) for a reaction with the following criteria: i. A three-step, endothermic reaction; ii. AG3 > 0 but AG₁ AG‡₁ >> AG‡₂. Which species does the structure of the transition state of the rate-determining step resemble?arrow_forwardConsider the following mechanism: Cl2 → Cl+ + Cl- Cl- + H2S → HCl + HS- Cl+ + HS- → HCl + S What is the role of Cl+? A. product B. intermediate C. catalyst D. reactantarrow_forward
- Consider the following mechanism: NO → N + O O + O3 → 2 O2 O2 + N → NO2 What is the role of O2? A. product only B. intermediate only C. Both intermediate and product D. reactant E. catalystarrow_forwarda. b. C. d. e. 1. If for the reaction a b d e aX → by → 9 the rate law is determined to be r=[X]¹[Y]º the order of the reaction is 0 increasing the concentration of Y will have no effect on the rate increasing the concentration of X will have no effect on the rate increasing the concentration of Y will increase the rate of the reaction there is no way to determine the value of k 9 thenarrow_forwardQUESTION 7 Match the part of the rate law with the correct term. 2 NO(g) + Cl2(g) --> 2 NOCI(g) 2. rate 3.0 mol2*s A 3.0 mol2*s A. Y rate constant vorder of reaction with respect to NO vorder of reaction with respect to Cl2 В. 1 C. 2 overall reaction order D. 3 QUESTION 8 2.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY