
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
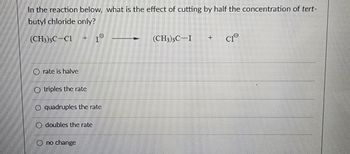
Transcribed Image Text:In the reaction below, what is the effect of cutting by half the concentration of tert-
butyl chloride only?
(CH3)3C-C1
rate is halve
H
Otriples the rate
O quadruples the rate
doubles the rate
no change
(CH3)3C I +
CIⓇ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- how do you draw the reductive elimination curved arrow mechanism for the inorganic reactionarrow_forwardA set of three nucleophilic displacement reactions is shown below: - Br -Br -Br "CHз Hзс CHз C В A NaCN CH-он SN2 reaction Which reaction (A, B, or C) proceeds the fastest? Which reaction (A, B, or C) proceeds the slowest?arrow_forwardDiscussion Prompt #3 CH3 H-Br Br CH3 Draw the mechanism for the reaction abovearrow_forward
- Rank the following starting materials in order of increasing rate of reaction (slowest to fastest) SN1 reaction: Lora OTos OTos OTos 3 O 2<1< 3 O1<3 < 2 O 3<1< 2 O1< 2 < 3 O2<3< 1 2.arrow_forwardAlcohols, ethers, epoxides Maximum allowed tries per question: Unlimited TSOH appears in many of these questions. It is a strong acid. Whenever you have a mechanism under acidic conditions, you can draw H+ instead of the entire acid. (27) Draw a mechanism for this reaction. Each set of intermediates must be enclosed in a rectangle, each box must be connected to the subsequent one with a reaction arrow, and each box (except the final one) should contain curved arrows showing the movement of electrons in each step. Launch MarvinJSTM viewer or click image to copy source OH +Harrow_forwardWhich of the following is true about the transition state below? HS----- H H -C-- H --Br 77 A) The H-C-H bond angle is 109.5⁰ (B) The distance between S and C atoms is longer than a typical covalent bond C) Pi bond formation is occurring (D) The product will have an (R) stereocenter E A methyl carbocation is formingarrow_forward
- answer fast correct answer pleasearrow_forward8. Which of the following depicts the energy profile of a nucleophilic substitution reaction that proceeds via SN2 mechanism? Doemos gniwollot ent to ribirwa Jenon s-slengie tertio cobized SAMMH Hedi ni (co A) MM (A Reaction Progress- Reaction Progress →> C) D) MM ↑ Lasgasgaizim ord short Reaction Progress blan Reaction Progress →arrow_forwardFor an E2 elimination reaction, if the concentration of the alkyl halide were cut in half, then the rate of the chemical reaction would: O Quadruple O Not change O Doutle O Be cut in haltarrow_forward
- Consider the pair of reactions. Draw the organic products, then predict the type of substitution mechanism and compare the expected rates. 30 R F Reaction 1: V Reaction 2: Draw product 1. Select Draw Templates More DDD :Ö: % 5 / |||||| C H F :Ö: T G F5 + B 6 :F :F: Y MacBook Pro H & 7 DMSO N H₂O F7 U J Erase 8 product 1 product 2 M DII F8 Draw product 2. Select Draw Templates More / |||||| C H ( 9 K F9 H O L F10 P F LL F11 3 + [ command option Erase F12 } 1arrow_forwardDraw step 4 of the mechanism. H₁₂C H3C- CH3 CH3 H- + -CH3 Edit Drawingarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY