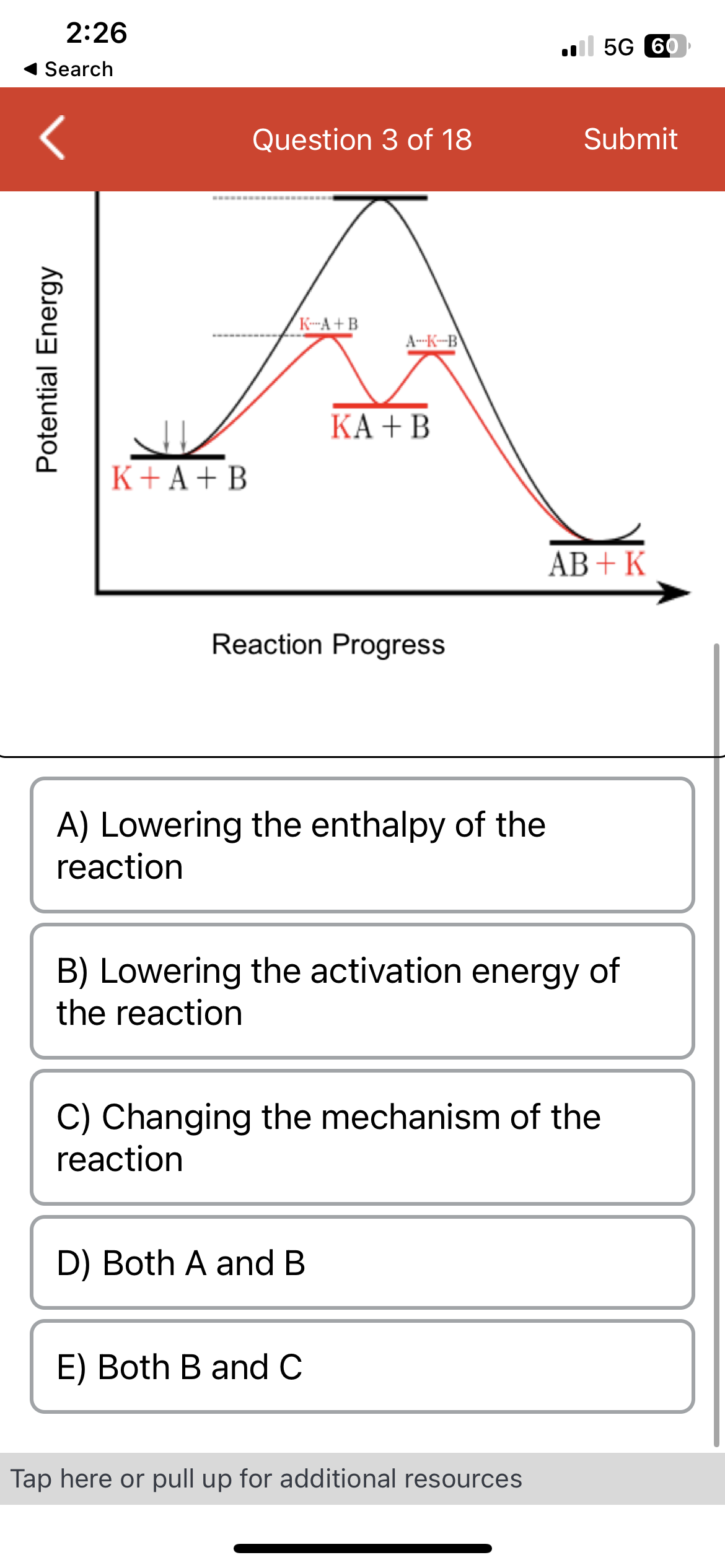
In the provided reaction profile, the black line represents the pathway of the reaction of A and B going to AB. The red line represents the pathway when catalyst K is added. What is catalyst K doing in the reaction? Potential Energy K + A + B AB K-A+B A-K-B KA + B AB+K
In the provided reaction profile, the black line represents the pathway of the reaction of A and B going to AB. The red line represents the pathway when catalyst K is added. What is catalyst K doing in the reaction? Potential Energy K + A + B AB K-A+B A-K-B KA + B AB+K
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter30: Capillary Electrophoresis, Electrochromatography, And Field-flow Fractionation
Section: Chapter Questions
Problem 30.9QAP
Related questions
Question
Transcribed Image Text:2:26
Search
Potential Energy
K+A+B
Question 3 of 18
K-A+B
A-K-B
Reaction Progress
KA + B
A) Lowering the enthalpy of the
reaction
D) Both A and B
E) Both B and C
B) Lowering the activation energy of
the reaction
C) Changing the mechanism of the
reaction
5G 60
Submit
Tap here or pull up for additional resources
AB + K
Transcribed Image Text:2:26
Search
Potential Energy
Question 3 of 18
K + A + B
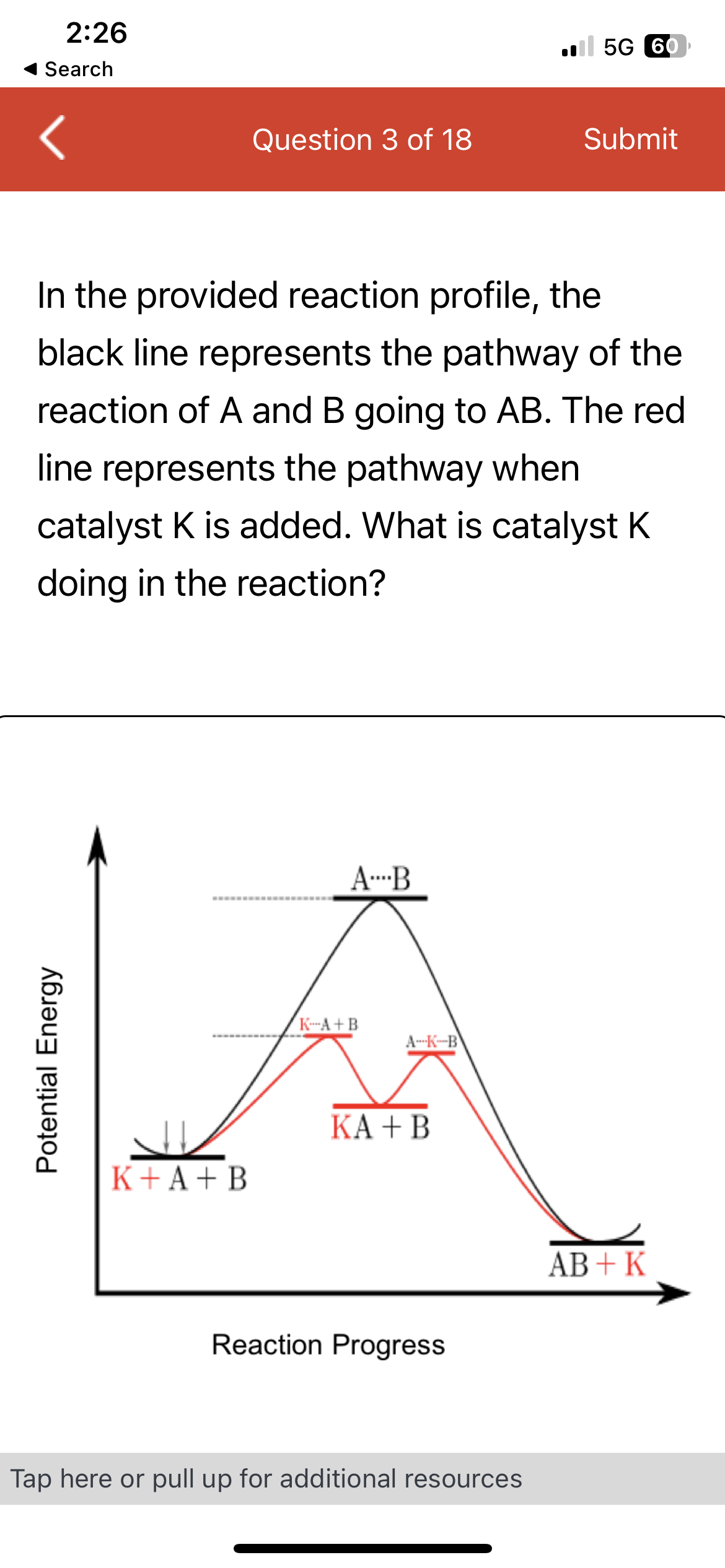
In the provided reaction profile, the
black line represents the pathway of the
reaction of A and B going to AB. The red
line represents the pathway when
catalyst K is added. What is catalyst K
doing in the reaction?
AB
K-A+B
A-K-B
KA + B
Reaction Progress
5G 60
Tap here or pull up for additional resources
Submit
AB+K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning