
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
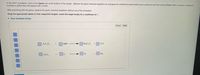
Transcribed Image Text:In the PHET simulation, click on the Game icon at the bottom of the screen. Balance the given chemical equations by changing the coefficients placed before each compound and then clicking Check. When a reaction is balanced
correctly a yellow face will appear with a smile.
After practicing with the game, balance the given chemical equations without use of the simulation.
Drag the appropriate labels to their respective targets. Leave the target empty for a coefficient of 1.
> View Available Hint(s)
Reset
Help
3
H,C,0.
NaOH
Na,C,0,
H,0
5
Fe, 0,
Fe
CO
7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Homework i ces - Balance the chemical equation. Note that you must select "1" if that is the correct coefficient, though the "1" is not typically written in a balanced equation, but is implied. 02 (Click to select) KNO3 → (Click to select) KNO2 + (Click to select) ✓ M 2 JUL 11 Saved 1 ******* 1 tv He Aarrow_forwardPlease answer Part Earrow_forward✓ Question Completion Status: QUESTION 16 In the following reaction, what is the correct coefficient for aluminum chloride? OA) 1 B) 2 ⒸC) 3 D) 4 Al + QUESTION 17 Cl2 → AIC13 Click Save and Submit to save and submit. Click Save All Answers to save all answers.arrow_forward
- Challenge 1 of 5 OH₂ + OF₂ Level: 1 Score: 0 Check Start Over 0 HF X+Y➡XY * * Balancing Chemical Equations PRET: Introduction Game Use the PhET simulation (use the introduction window above) to answer the following questions How many moles of ammonia is generated in the reaction between N₂ and H₂ How many moles of water is required to produce one mol of O₂ in water decomposition How many moles of O₂ is required in the combustion reaction of methane, CH4arrow_forwardImage uploaded answer is strictly prohibited please don't upload any image No answer from Chat GPTarrow_forwardNTS GAMES VIDEOS VA SOL PROG Balancing Act! Balance this equation! (Equation number 1 of 10) C6H6OS + 02 → 12CO2 + H2O + 2SO3 Check my answer!arrow_forward
- One of them is sorted incorrectlyarrow_forwardQuestion 10 Listen If you have not weighed out enough solid compound on the balance, you should... a) pour more compound in your hand and then add it to the weigh boat on the balance. b) throw out the sample in the trash and start over. c) remove the weigh boat and add more compound, then reweigh the sample. d) throw out the sample in the waste container and start over. e) pour more compound in the weigh boat on the balance.arrow_forwardMISSED THIS? Watch KCV Limiting Beactant. Theoretical Yield and Percent Yield WE Limiting Reactant and Theoretical Yield Read Section 4.4. You can click on the Review link to access the section in your eText Find the limiting reactant for each initial amount of reactants 2Na(s) + Bra(g)-> 2NaBr(s) Part A 7 mol Na and 3 mol Bry Express your answer as a chemical formula. View Available Hint(s) Hint 1. Identify conversion factors between the numbers of moles of Na and NaBr and between the numbers of moles of Bry and NaBr ΑΣΦΑ ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY