
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
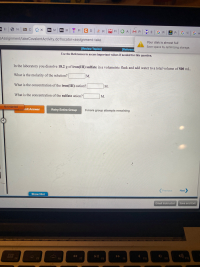
Transcribed Image Text:In the laboratory you dissolve 18.2 g of iron(III) sulfate in a volumetric flask and add water to a total volume of 500 mL.
What is the molarity of the solution?
М.
What is the concentration of the iron(III) cation?
М.
What is the concentration of the sulfate anion?
M.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the laboratory you dissolve 24.3 g of potassium hydroxide in a volumetric flask and add water to a total volume of 375 mL. What is the molarity of the solution? 0.433 M. What is the concentration of the potassium cation? 1.16 M. What is the concentration of the hydroxide anion? 1.16 M. An error has been detected in vour answer Cherkarrow_forward2. Your 2- year old baby brother has a fever. The doctor prescribed a medicine and it should be prepared by adding amount of water. The amount of powder to be dissolved is 0.500 mol in 50.0 mL of distilled water. How will you express the concentration of the medicine? *arrow_forwardIt required 66.66 mL of 2.000 M HBrO3 to titrate (neutralize) 50.00 mL of an Al(OH)3 solution. What is the molarity, M, of the aluminum hydroxide solution ? 3 HBrO3 + Al(OH)3 3 H20 + Al(BrO3)3 А. О.8888 М B. 0.2666 M C. 0.08888 M D. 0.02666 Marrow_forward
- A chemist wants to determine the concentration of an acidic solution. They start by placing 25.0 mL of the acid in a flask. Then, they titrate it with 13.0 mL of 0.10 M NaOH. a. How many moles of NaOH were added to the acid? b. Assuming the acid and base react in a 1:1 ratio, how many moles of acid are in the flask? c. What is the molarity of the unknown acid?arrow_forward6. Sodium sulfide reacts with hydrochloric acid to produce hydrogen sulfide and sodium chloride. How many grams of sodium sulfide are required for complete reaction with 15.0 mL of 0.250 M hydrochloric acid? The molar mass of sodium sulfide is 78.046 g/mol. (Hint: start by writing the balanced equation for the reaction.)arrow_forwardsentences. 3. Virtual Lab Questions a. A student prepares a solution of hydrochloric acid that is approximately 0.1 M and wishes to determine its exact concentration. A 25.00 mL portion of the HCI solution is transferred to a flask, and after a few drops of indicator are added, the HCI solution is titrated with 0.07575 M NaOH solution. The titration requires exactly 38.92 mL of the standard NaOH solution to reach the end point. What is the molarity of the HCI solution? b. It takes 46.22 mL of a 1.021 M NaOH solution to neutralize a solution of 4.4567 g of an unknown monoprotic acid in 80.00 mL of water. Calculate the molecular weight of the acid. 4(D) 5arrow_forward
- 1. A 12.5 mL volume of 0.500 M H2SO4 neutralizes 50.0 mL of NaOH. A. What is the acid (formula and name)? B. What is the base (formula and name)? C. Write a balanced chemical equation for the reaction. D. Write the complete ionic equation for the reaction. E. Write the net ionic equation for the reaction. F. What is the concentration of the NaOH solution? (This is a titration).arrow_forwardA student weighs out a 2.76 g sample of NaOH , transfers it to a 500. mL volumetric flask, adds enough water to dissolve it and then adds water to the 500. mL tick mark. What is the molarity of sodium hydroxide in the resulting solution? Molarity = _____Marrow_forward1. What is the mass of the solution when 50.0 mL of 1.0 M HCI solution is mixed with 50.0 mL of 1.0 M NaOH solution? (d = 1.02 g/mL )arrow_forward
- Calculate the molarity of 150.0 mL of sodium hydroxide solution needed to react with 65.7 mL of a 0.128 M solution of phosphoric acid. OA. 0.877 M Ов.0.168 м Oc. 0.0974 M OD. 0.0420 M OE. 0.0187 M OF. 0.0390 M OG. 0.0561 M OH.0.292 M QUESTION 2 What volume of 0.128 M solution of hydrochloric acid is needed to react with 200.0 mL of 0.128 M calcium hydroxide? O A. 0.0000410 mL Ов. 100. mL OC. 0.000164 mL OD. 24400 mL OE. 400. mL OF. 0.0000819 ml OG. 200. ml OH.6100 mL QUESTION 3 Given a density of 1.17 g/mL, what volume of a 50.5% solution of sulfuric acid is needed to react 350.0 mL of 0.138 M strontium hydroxide? OA. 95.6 mL Ов. 13100 mL OC. 5.15 mL OD.6.02 mL OE. 9.38 mL OF. 8.02 mL OG. 15300 mL OH.4.40 mL QUESTION 4 Given 200.0 mL of a 0.139 M solution of aluminum chloride, according to the following equation, how many mL of a 0.358 M solution of sodium carbonate are needed to react with the aluminum chloride? 3 NazcOg(aq) + 2 AICI3(aq) - Alg(coz)a(s) + 6 Naci(aq) OA. 77.7 ml OB.…arrow_forwardIn the laboratory you dissolve 22.7 g of potassium bromide in a volumetric flask and add water to a total volume of 250 mL. What is the molarity of the solution? М. What is the concentration of the potassium cation? М. What is the concentration of the bromide anion? М.arrow_forward3. Nitric acid reacts with potassium hydroxide. a. Write a balanced chemical equation to describe this reaction b. 50.0 mL of a nitric acid solution is titrated with 48.6 mL of 0.124 M potassium hydroxide solution. i. Calculate the moles of potassium hydroxide used for the titration. 50.0mL ii. Calculate the molarity of the 50mL nitric acid solution before the reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY