
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
3
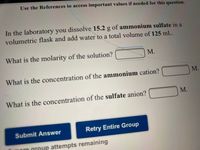
Transcribed Image Text:Use the References to access important values if needed for this question.
In the laboratory you dissolve 15.2 g of ammonium sulfate in a
volumetric flask and add water to a total volume of 125 mL.
What is the molarity of the solution?
М.
M.
What is the concentration of the ammonium cation?
М.
What is the concentration of the sulfate anion?
Submit Answer
Retry Entire Group
0 greyp attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- What are advantages and limitations of different types of modelling? (modelling with experiments, computer simulations, modelling kits and quantitative modelling)arrow_forwardWhich piece of equipment would you use to measure 7.10 mL of CuSO4 (aq)?arrow_forward(4pts) Determining the Thickness of Aluminum Foil Be sure to report answers with the correct number of significant figures. Length:3.60 cm Width: 3.11 cm Mass: 0.521 g (2pts) Volume of aluminum foil (cm³) (2pts) Thickness of aluminum foil (cm)arrow_forward
- The world population is estimated to be 7.4 x 109. Nauru is the smallest island nation and comprises 1.5 x 10-4% of the world population. If the percentage of left-handed people is approximately 12%, estimate the number of left-handers on the island of Nauru.arrow_forward7. A cube of an unknown metal measures 1.69 mm on one side. The mass of the cube is 31.4 mg. Which of the following is most likely the unknown metal? SHOW your work [5 points] Metal Density (g/ cm- cm3) rhodium 12.4 8.96 copper niobium 8.57 vanadium 6.11 zirconium 6.51arrow_forwardThe most common oxide of vanadium, V205, is used as a catalyst in manufacturing sulfuric acid H2 SO4. The density of vanadium is 6.00 3 g/cm. Express this in SI units ( 3 kg /m³). Density 3 kg/m³arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY