
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:P.
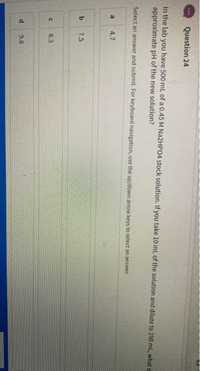
Question 24
In the lab you have 500 mL of a 0.45 M NA2HPO4 stock solution. If you take 10 ml. of the solution and dilute to 250 ml, what is
approximate pH of the new solution?
Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer.
a
4.7
7.5
8.3
9.8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help with questions 1& 2arrow_forwardcalculate the pH of 20.00mL of a solution of acetic acid, HC2H3O2 0.105M with NaOH 0.105 M. For acetic acid, Ka = 1.8x 10 ^ -5 a) initial pHb) After adding 5.00 mL of NaOHc) After adding 6.00mL of NaOH please give me step-by-step solution and circle answer pleasearrow_forwardMy Questions b X VCU SONA FAFSA: Apply f X + X edugen/Iti/main.uni Return to Blackboard S Kime, Explorations in College Algebra, 6e Help | System Announcements PRINTER VERSION BACK NEXT Chapter 6, Algebra Aerobics 6.3, Question 05 A typical pH value for rain or snow in the northeastern United States is about 4.0. Is this basic or acidic? What is the corresponding hydrogen concentration? How does this compare with the hydrogen concentration of pure water? Round your answers to two decimal places when necessary Type: Hof the rain: x10-5 x10. This is H of pure water: than the concentration of the rain. LINK TO TEXT By accessing this Question Assistance, you will learn while you earn points based on the Point Potential Policy set by your instructor. SUBMIT ANSWER SAVE FOR LATER Question Attempts: 0 of 5 used Earn Maximum Points available only if you answer this question correctly in three attempts or less. % powered by MapleNet 7:07 PM 10/14/2019 re to searcharrow_forward
- A solution has a pH of 8. Which best describes the solution? a strong acid a strong base a weak acid a weak base Save and Exit Next Mark this and return .com/ContentViewers/AssessmentViewer/Activitarrow_forwardWhat is the pH of a 9.34 x 10-5 M HCIO4 solution? (the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation--examples include 1.23 and 12.3 and -123 and 123. and 120.) MacBook Pro esc ! @ # $ % & 8 %3D 1 2 3 4 6. 7 { Q W E R T. Y F J K A C V command option ontrol option command .. .. Varrow_forwardYou are given a 0.25 M solution of an unknown weak base. Use 1/10th of dilution to find unknown weak base The diluted solution has a pH= 11.2. a) Write out an equation shows the reaction of base + waterb) What is the Ka and KB of weak basearrow_forward
- Both answer with explanation please.arrow_forwardWhat is the pH when 20.0 mL of 0.10 M sodium hydroxide is added to 40.0 mL of 0.15 M HIO (Ka = 2.0 × 10–11) Group of answer choices 10.40 11.60 7.54 10.70arrow_forwardPart A Consider two solutions, solution A and solution B. [H] in solution A is 430 times greater than that in solution B. What is the difference in the pH values of the two solutions? Express your answer using two decimal places. ApH= v %3D Submit Request Answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY