
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
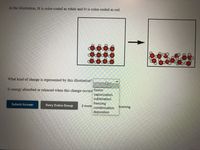
Transcribed Image Text:In the illustration, H is color-coded as white and O is color-coded as red.
What kind of change is represented by this illustration?
Is energy absorbed or released when this change occurs' fusion
vaporization
sublimation
freezing
condensation
Submit Answer
Retry Entire Group
2 more
maining
deposition
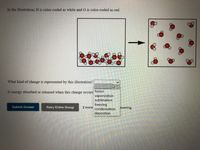
Transcribed Image Text:In the illustration, H is color-coded as white and O is color-coded as red.
What kind of change is represented by this illustration?
Is energy absorbed or released when this change occurs'
fusion
vaporization
sublimation
freezing
Submit Answer
Retry Entire Group
2 more
maining
condensation
deposition
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help me solve the following question, explain and make sure 1000% its correct thanksarrow_forwardPlease don't provide handwriting solutionarrow_forwardMatch each term to the correct example. Quartz (continuous chain of silicon dioxide) v [ Choose] lonic solid Solid water Network covalent solid Nonbonding atomic solid Metallic solid Potassium lodide salt Amorphous solid Molecular solid Nickel Solid radon [ Choose]arrow_forward
- cture Assignment Chapter 06 on-Changes of State Part B When a solid is placed in a container and heat is applied, a phase change occurs. Watch the video and sort the parts of the curve based on whether the average energy of the molecules is changing, or is constant. > View Available Hint(s) Reset Help The solid is heated at the melting The liquid is heated to reach the boiling point The solid is heated to reach the point melting point The liquid is heated at the boiling point Average molecule energy change Average molecule energy constant ... 1,098 16 MAR átv MacBook Air esc 80 000 DD F1 F2 F3 F4 F5 F6 F7 F8 F9 F11 F12 F19arrow_forwardThe vapor pressure of liquid diethyl ether, C₂H5OC₂H5, is 100 mm Hg at 262 K. A sample of C₂H5OC₂H5 is placed in a closed, evacuated 527 mL container at a temperature of 262 K. It is found that all of the C₂H5OC₂H5 is in the vapor phase and that the pressure is 52.0 mm Hg. If the volume of the container is reduced to 314 mL at constant temperature, which of the following statements are correct? Choose all that apply. ☐ The pressure in the container will be 100 mm Hg. Liquid diethyl ether will be present. Some of the vapor initially present will condense. No condensation will occur. Only diethyl ether vapor will be present.arrow_forwardHow are sublimation and evaporation the same and how are they different? Edit View Insert Cormesarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY