
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
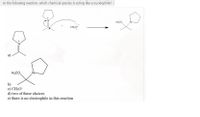
Transcribed Image Text:In the following reaction, which chemical species is acting like a nucleophile? [
H,CO
CH,O
H3CO
b)
c) CH;O¯
d) two of these choices
e) there is no electrophile in this reaction
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (please show reaekson and question information and correct answer )arrow_forwardConsider the reduction of 4-nitrobenzaldehyde using sodium borohydride, NaBH4 in ethanol: what happen at initial step 1) a hydride ion attacks the carbonyl group's carbon atom 2)a hydride ion attacks the nitro group's nitrogen atom 3) a sodium ion attacks the carbonyl groups oxygen atom 4) a sodium ion attacks a nitro group's oxygem atomarrow_forwardQ A F1 2 Question 13 Consider the reaction below. [1] Draw curved arrows to show the movement of electrons in each step. (Draw in missing lone pair electrons if they are directly involved in a step). [3] Identify the mechanistic pattern in each step (nucleophilic attack, proton transfer, loss of leaving group, rearrangement). [2] Draw the transition states for step 1 and step 2. W S F2 3 Step 1 80 F3 E D H₂O this response. $ 4 F4 CI- Step 2 R % 5 F 0 F5 T G Harrow_forward
- H FCEC Organic product Inorganic product + + H Br Draw the organic product. Draw the inorganic product. Select Draw Rings More Erase Select Draw Rings More Erase H Br H Br Select the statement that properly identifies the nucleophile, substrate, and leaving group. O Br is the substrate, CH,C=C:- is the nucleophile, and CH, Br is the leaving group. O CH,C=C: is the substrate, CH, Br is the nucleophile, and Br is the leaving group. O CH, Br is the substrate, CH,C=C: is the nucleophile, and Br is the leaving group.arrow_forwardWould you say its sn1 ir sn2, I said it was sn1,because the nucleophile is weak, but my teachers said it was sn2arrow_forwardQUESTION 33 Which of the following rate laws accurately describes the rate determining step in an Et elimination of alcohols? OA Rate - Mearbocationproton OH. Rate = kfoxonium lon)[alcohol O cRate kfcarbocation] OD Rate = loxonium lon] OE Rate kcarbocation][water) QUESTION 34 Aqtuieous KMnO4 which is purple, indicatos the presence of alkenos by reacting with them to forn coioriess precipitate and OA. none of the answers OR 12 diols, molybdenum dioxide, orange Or vicinal diols, manganese dioxide. brown O D. glycols, magnesium dioxide, brown OE alkyl chlorides, potasslum hydroxide, yellow O F. geminal diols, manganese dioxide, brown QUESTION 35 five carbon (S)-(+) Carvone was isolated from caraway seeds and belongs to a group of natural compounds called O steralds, one pentane O alkaloids, three, tert-butyl O terpenoids, two, isoprene which contain subunit(s) pentanoids, one, pentane O None of the abovearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY