
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
In the condenser of a thermoelectric plant, steam will be condensed at a temperature of 50°C by using cooling water from a nearby lake, which enters the condenser tubes at 18°C at a rate of 101 kg/s and leaves at 27°C Determine the rate of condensation of the vapor in the condenser.

Transcribed Image Text:In the condenser of a thermoelectric plant, steam will be condensed
at a temperature of 50°C by using cooling water from a nearby lake,
which enters the condenser tubes at 18°C at a rate of 101 kg/s and
leaves at 27°C Determine the rate of condensation of the vapor in the
condenser.
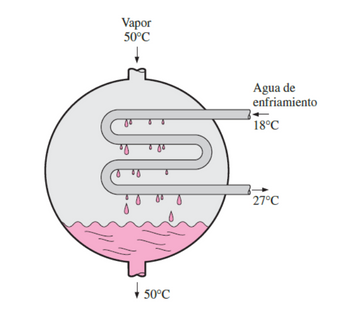
Transcribed Image Text:Vapor
50°C
50°C
Agua de
enfriamiento
18°C
27°C
Expert Solution
arrow_forward
Step 1
The principle of heat balance is used to solve the problem that is heat absorbed by the lake water will be equal to the heat released by steam.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- An autoclave contains 1000 cans of pea soup. It is heated to an overall temperature of100C. If the cans are to be cooled to 40C before leaving the autoclave, how much coolingwater is required if it enters at 15C and leaves at 35C? The specific heats of the pea soupand the can metal are 4.1 kJ/kgC and 0.5 kJ/kgC, respectively. The weight of each can is60g and it contains 0.45 kg of pea soup. Assume that the heat content of the autoclave wallsabove 40C is 1.6x104 kJ and that there is no heat loss through the walls. Ans. 1,526.85 Kgarrow_forwardA rigid, insulated tank that is initially evacuated is connected through a valve to a supply line that carries Helium at 200 kPa and 120°C. Now the valve is opened, and helium is allowed to flow into the tank until the pressure reaches 200 kPa, at which point the valve is closed. Determine the final temperature of the Helium in the tank if the specific heat ratio for Helium (k=Cp*/Cv*) is 1.667.arrow_forwardSteam enters the condenser of a steam power plant at 20 kPa and aquality of 90 percent with a mass flow rate of 20,000 kg/h. It is to be cooled by water from anearby river by circulating the water through the tubes within the condenser. To preventthermal pollution, the river water is not allowed to experience a temperature rise above10°C. If the steam is to leave the condenser as saturated liquid at 20 kPa, Determine(a) the mass flow rate of the cooling water required, and(b) rate of heat loss from the steam. Please attempt (a) then (b)arrow_forward
- 5-84 Air (Cp=1.005 kJ/kg°C) is to be preheated by hot exhaust gases in a cross-flow heat exchanger before it enters the furnace. Air enters the heat exchanger at 95 kPa and 20°Ct at rate of 0.6 m³/s. The combustion gases (Cp=1.10 kJ/kg°C) that enter at 160°C at a rate of 0.95 kg/s and leave at 95°C. Determine the rate of heat transfer to the air and its outlet temperature. Air 95 kPa 20°C 0.6 m³/s Exhaust gases 0.95 kg/s 95°Carrow_forwardCold water enters a steam generator at 20°C and leaves as saturated vapor at 200°C. Determine the fraction of heat used in the steam generator to preheat the liquid water from 20°C to the saturation temperature of 200°C.arrow_forward2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY