Question
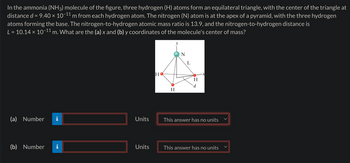
Transcribed Image Text:In the ammonia (NH3) molecule of the figure, three hydrogen (H) atoms form an equilateral triangle, with the center of the triangle at
distance d = 9.40 × 10−11 m from each hydrogen atom. The nitrogen (N) atom is at the apex of a pyramid, with the three hydrogen
atoms forming the base. The nitrogen-to-hydrogen atomic mass ratio is 13.9, and the nitrogen-to-hydrogen distance is
L = 10.14 × 10-11 m. What are the (a) x and (b) y coordinates of the molecule's center of mass?
(a) Number
Units
(b) Number
N
L
HO
H
d
H
This answer has no units
Units
This answer has no units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 6 images

Knowledge Booster
Similar questions
- A lottery machine uses blowing air to keep 2000 Ping-Pong balls bouncing around inside a 1.0m×1.0m×1.0m box. The diameter of a Ping-Pong ball is 3.0 cm. What is the mean free path between collisions? Give your answer in cm.arrow_forwardManufacturers of headache remedies routinely claim that their own brands are more potent pain relievers than the competing brands. Their way of making the comparison is to compare the number of molecules in the standard dosage. Tylenol uses 325 mg of acetaminophen (C8H9NO2) as the standard dose, while Advil uses 2.00 x 102 mg of ibuprofen (C13H1802). Find the number of molecules of pain reliever in the standard doses of (a) Tylenol and (b) Advil. (a) Number Units (b) Number Unitsarrow_forwardThe vapor pressures of the components, A and B, in a binary solution have been modeled and found to obey хара exp(0.75 XB) A A exp(0.75x) where XÃ and are the mole fractions, and PA* and PB* are the vapor pressures of each pure substance at room temperature. (a) If PA* = 0.084 bar and the total pressure of a mixture with XA = 0.40 is 0.125 bars, what is PB*, the vapor pressure of pure B (in bars)? P = X P А P₁ = X_P B QUESTION 14 B B * * Continuation of the previous problem (b) Assuming that the vapor is an ideal gas, what is the mole fraction of component B in the vapor phase?arrow_forward
- 8.85 x 10-12 C2 Useful Constants: k = 9.00 x 10° Nm2 C2 %3D €0 = Nm2 e = 1.6 x 10-19C %3D me = -31 kg = 9.11 × 10 31 kgarrow_forwardViscosity of fluid plays a significant role in the analyses of many fluid dynamics problems. The viscosity of water can be determined from the following correlation: q10(72,) where e = viscosity (N/s•m²) T- temperature (K) - 2.414 x 10-s 2 - 247.8 (K) s = 14 0 (K) What is the appropriate unit for q, if the above equa- tion is to be homnogeneous in units?arrow_forwardAt what temperature would the rms speed of hydrogen atoms equal the following speeds? (Note: The mass of a hydrogen atom is 1.66 x 10-27 kg.) (a) the escape speed from Earth, 1.12 x 104 m/s K (b) the escape speed from the Moon, 2.37 x 10³ m/s Karrow_forward
- In a gas that is a mixture of water (two H and one O) and carbon dioxide (one C and two O), if the water has average velocity of 347 m/s, what is the average velocity of the carbon dioxide molecule? Give your answer as number in units of m/s without entering the units.arrow_forward25. A movable circular lid rests on top of an a gas-filled a cylinder; the cylinder and lid have a radius of 12.0 cm; the lid has a weight of 800 N. Outside the cylinder is atmospheric pressure, 1.01 x 105 Pa. Inside the cylinder are 6.0 moles of argon gas. The gas temperature is 400 °K. What is the volume of the gas (in cubic meters)? The atomic mass of argon is 39.95. (a) 0.04 (b) 0.21 (c) 0.17 (d) 0.13 (e) none of these osodilo 0 (0) 2 (0) SC on (s)arrow_forwardPd Pd 1. Let's consider a toy model of nuclear fission. Suppose an nucleus of Uranium-235 (92 protons, molar weight of 235 g/mole) "splits" into two "daughter" nuclei of Palladium (46 protons each) – this is not how it really happens, but it's a very simple model that actually gives fairly accurate results. The radius of the original U-235 nucleus is about 7.4 x 10-15 m. (a) If the Pd nuclei each have half the volume of the U nucleus, which is reasonable, and they are "touching" right after the split, how far apart are their centers? (b) Using conservation of energy, what will be the sum of the kinetic energies of the Pd nuclei when they are far apart from each other? (c) That's energy of one atom undergoing fission, so what, then, is the energy released by the fission of 1 kg of U-235? Express this in Joules and also in kilotons of TNT, where 1 kt = 4.2x1012 J. (The Hiroshima bomb yielded about 15 kt) (d) How many kwh (kilowatt-hours) of energy is this, (1 kwh = 3.6x10° J), and (if…arrow_forward
arrow_back_ios
arrow_forward_ios