
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
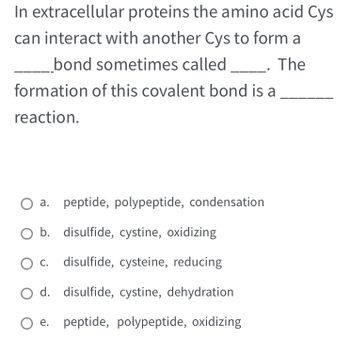
Transcribed Image Text:In extracellular proteins the amino acid Cys
can interact with another Cys to form a
__bond sometimes called ________. The
formation of this covalent bond is a
reaction.
a.
O b. disulfide, cystine, oxidizing
c. disulfide, cysteine, reducing
d.
disulfide, cystine, dehydration
O e. peptide, polypeptide, oxidizing
peptide, polypeptide, condensation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The bonds that are important inthe secondary structure of a protein are a)hydrogen bonds b.peptide bonds c.hydrophobic interaction d.salt bridges e.disulfide bondsarrow_forwardThe side chain of which residue can be hydrogen bond donor: a. Thr b. Ala c. Ile d. Pro e. Phearrow_forwardWhich of the following is NOT found in RNA? A. thymine B. adenine C. uracil D. cytosine E. guaninearrow_forward
- Which of the following amino acid or types of amino acids are are rarely found in beta sheets? a. Polar charged amino acids b. Polar uncharged amino acids c. Non polar amino acids D. Glycinearrow_forwardWhich of the following is incorrect about the beta-sheet? a. The strands in anti-parallel beta-sheets are usually connected with turns b. Side chains are alternated above and below the sheet C. Parallel beta-sheets are less stable than the anti-parallel ones Od. It is stabilized by hydrophobic interactions between the side chains of nonpolar residuesarrow_forwardThe following are functions of lipids EXCEPT A. Isolate cellular components from its environment B. Serve as signal molecules and mediators of cellular processes C. Membrane fluidity determinant D. Alternative energy source of cells O E. Facilitate movement of metabolites in and out of cellsarrow_forward
- Which of the following is incorrect about the peptide Arg-Asp-Cys-Tyr-Gln-Arg-Glu? It has six peptide bonds b. It has a net charge of zero at pH 7 It has six charged groups at pH 7 d. None; all the other choices are correct a. C.arrow_forwardHow would adding acid to a non-buffered solution be most likely to affect protein structure? Select one: a. Adding acid would disrupt the primary structure of the protein b. Adding acid would cause the protein to become a lipid c. Adding acid would cause the protein to become a carbohydrate d. Adding acid would disrupt the secondary and tertiary structure of the proteinarrow_forwardAn allosteric interaction between a ligand and a protein is one in which: a. binding of a molecule to a binding site affects binding of additional molecules to the same site. b. binding of a molecule to a binding site affects binding properties of another site on the protein. c. binding of the ligand to the protein is covalent. d. multiple molecules of the same ligand can bind to the same binding site. e. two different ligands can bind to the same binding site.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON