
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
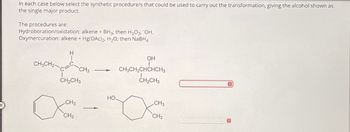
Transcribed Image Text:In each case below select the synthetic procedure/s that could be used to carry out the transformation, giving the alcohol shown as
the single major product.
The procedures are:
Hydroboration/oxidation: alkene + BH3; then H2O2, OH.
Oxymercuration: alkene + Hg(OAc)2, H₂O; then NaBH4
H
CH3CH2
CH3
CH2CH3
OH
CH3CH2CHCHCH3
CH2CH3
HO.
CH3
CH3
CH3
CH3-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the structural formula(s) for the major product(s) of each of the following reactions. Unless required, ignore stereochemical detailsarrow_forward4. Synthesis Using the carbon-containing starting material(s), propose a synthesis by drawing structures for all intermediates. The carbon atoms in the product must originate from the starting material(s) (or a carbene/carbenoid or CO2), but you may use as many equivalents of each starting material as you would like, and any reagent/reaction you know. (note: no mechanisms are required). OHarrow_forward3. Provide the order of addition of reagents added for the following conversions in the correct order. O₂N CH3arrow_forward
- 5e.arrow_forwardProvide the major product(s) of each reaction below. Show the stereochemistry of each stereocenter (for example, by using wedge-dash structures for chirality centers and clear bond angles for alkenes). For enantiomers, you only need to draw one of the enantiomers. Br2, H₂O 1) 03 2) MeSMe H₂, Pd/C HgSO4 H₂SO4 H₂O 1) BH3 2) NaOH, H₂O2 H₂O 1) 9-BBN 2) NaOH, H₂O2 H₂Oarrow_forwardPlease help me answer these organic chemistry questions. Explanations are welcome/arrow_forward
- Provide the organic product(s) formed in the highest yield for each reaction below. If more than one product is provided, label the product formed in the highest yield "MAJOR" if it is possible to determine. Include counterions to balance charges in your answer and indicate stereocenters whenever appropriate. HO D OH xes N cho ia NaOH/H₂O, 100 °C 1. NaNH2 (2 equiv.) THF, 0->65 °C 2. dilute H30Ⓡ CH3CH₂ONa (catalytic) CH3CH₂OH, 78 °C NaOH H₂O, 100 °Carrow_forward2. Give the products when 1-methyl-2-deutero-cyclohexene is treated with the following reagents. Be sure to clearly show stereochemistry of product(s). 1) Hg(OAc)2, H,о 2) NABH, CCI, (an inert solvent) CH3 H2, Pd/C ELOH CH;OH H,SO,(cat.)arrow_forwardThe following tertiary alkyl bromides undergo an SN1 reaction in aqueous acetone to form the corresponding tertiary alcohols. Rank the alkyl bromides from most reactive to least reactive.arrow_forward
- Draw structural formulas for all alkenes that could be used to prepare the alcohol shown below by oxymercuration.arrow_forward6- In a 1H-NMR spectra on a scale of 0 – 10 ppm, Show the exact locations in ppm of 1-the saturated hydrocarbons, 2- the unsaturated hydrocarbons, 3- the halogenatedhydrocarbons and 4- the aromatic hydrocarbons.arrow_forwardDraw structural formulas for all alkenes that could be used to prepare the alcohol shown below by oxymercuration.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY