
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
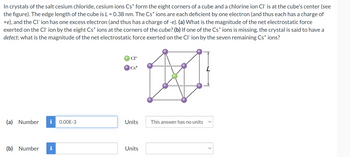
Transcribed Image Text:In crystals of the salt cesium chloride, cesium ions Cs+ form the eight corners of a cube and a chlorine ion Cl is at the cube's center (see
the figure). The edge length of the cube is L = 0.38 nm. The Cs+ ions are each deficient by one electron (and thus each has a charge of
+e), and the Cl ion has one excess electron (and thus has a charge of -e). (a) What is the magnitude of the net electrostatic force
exerted on the Cl ion by the eight Cs+ ions at the corners of the cube? (b) If one of the Cs+ ions is missing, the crystal is said to have a
defect; what is the magnitude of the net electrostatic force exerted on the CI ion by the seven remaining Cs+ ions?
CI-
Cs
(a) Number
0.00E-3
Units
This answer has no units
(b) Number i
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- In the figure three identical conducting spheres initially have the following charges: sphere A, 6Q: sphere B, -8Q; and sphere C, 0. Spheres A and B are fixed in place, with a center-to-center separation that is much larger than the spheres. Two experiments are conducted. In experiment 1, sphere C is touched to sphere A and then (separately) to sphere B, and then it is removed. In experiment 2, starting with the same initial states, the procedure is reversed: Sphere C is touched to sphere B and then (separately) to sphere A, and then it is removed. What is the ratio of the electrostatic force between A and B at the end of experiment 2 to that at the end of experiment 1? Number i Units C Barrow_forwardThree charged marbles are glued to a nonconducting surface and are placed in the diagram as shown. The charges of each marble are q₁ = 6.70 µC, 92 = 1.01 µC, and q3 = -2.27 μC. Marble q₁ is a distance r₁ = 3.00 cm to the left of the marble 92, while marble q3 is a distance r3 = 2.00 cm to the right of the marble q2, as shown. Calculate the magnitude of the electric field a distance r' = 1.00 cm to the left of the center marble. N/C y K t * 91 92 observation point ---Select--- V 93 Another marble is placed 1 cm to the left of the middle marble. If this new marble has a charge of -3.71 µC, calculate magnitude and direction of the force on it. magnitude direction N 7₂=0marrow_forwardWater has a mass per mole of 18.0 g/mol, and each water molecule (H2O) has 10 electrons.(a) How many electrons are there in 3.56 liters of water? (A liter is 1.00 10-3 m3)electrons(b) What is the net charge of all these electrons?Carrow_forward
- A small metal sphere carrying a net charge of Q = -2.00 uC is held stationary by insulating supports. A second small metal sphere with a net charge of Q 2 = +7.80 uC and mass 1.50 g is is held 0.8 m away from the center of the first sphere. Assume the two spheres can be treated as point charges and that you can ignore gravity. If the second sphere is released, how fast will it be traveling when it is 0.6 m away from the center of the first sphere?arrow_forwardA solution contains 5.62E11 Cl– ions and 1.35E11 Ca2+ ions. What is the total net charge in the solution (in Coulombs)?arrow_forwardThree charged marbles are glued to a nonconducting surface and are placed in the diagram as shown. The charges of each marble are q = 6.30 µC, 92 = 1.93 pC, and 93 = -2.20 pC. Marble q, is a distance r = 3.00 cm to the left of the marble q2, while marble q3 is a distance r3 = 2.00 cm to the right of the marble q2, as shown. Calculate the magnitude of the electric field a distancer = 1.00 cm to the left of the center marble. N/C observation point Another marble is placed 1 cm to the left of the middle marble. If this new marble has a charge of -3.60 pC, calculate magnitude and direction of the force on it. magnitude direction -Select varrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON