
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:O(a) 1x1010; (b) 1x104/s
(a) 1.25x10-5; (b) 5x10-11/s
O(a) 1x1010; (b) 5x10-11/s
O(a) 2x1014; (b) 1.25x10-5
(a) 2x10-14; (b) 1x104/s
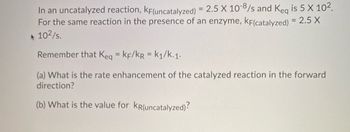
Transcribed Image Text:In an uncatalyzed reaction, KF(uncatalyzed) = 2.5 X 10-8/s and Keq is 5 X 10².
For the same reaction in the presence of an enzyme, KF(catalyzed) = 2.5 X
10²/s.
Remember that Keq = KF/KR = k₁/k-1.
(a) What is the rate enhancement of the catalyzed reaction in the forward
direction?
(b) What is the value for KR(uncatalyzed)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Consider an enzyme that catalyzes the reaction S2 P, by the following simple reaction mechanism: k, E + S 2 E•S →E kcat + P Suppose the enzyme acquires a mutation that causes k1 to be 10-times smaller than for the wild-type (non-mutant) enzyme. Suppose you measure the initial reaction rate (vo) at several different [S] for the mutant and the wild-type enzymes. Under what conditions would the mutation have a greater effect on the reaction rate (vo) of the mutant enzyme compared to the wild-type enzyme - at very low [S], or at very high [S]? Explain briefly how you decided.arrow_forwardIn the scheme below which represents the mechanism of action for a large number of enzymes: A+B⟺AB⟶C The steady state approximation is reached when: d[AB]/dt≈0 k2≫k1 k−1≫k1 k−1=k1arrow_forwardAn experiment was carried out to measure the reaction rate of hydrolysis of acetylcholme (substrate) with serum enzymes (Eadie, 1949). In the experiment, two experiments were conducted, namely experiment 1 without using a prostigmine inhibitor and experiment 2 using a prostigmine inhibitor at 1.5 x 10^-7 mol/l. the data obtained are: a. Is prostigmine competitive or noncompetitive inhibitor? b. determine the value of km and rmax for the two experiments, comparearrow_forward
- The Michaelis-Menten equation models the hyperbolic relationship between [S] and the initial reaction rate V₁ for an enzyme-catalyzed, single-substrate reaction E + S ⇒ ES →→ E + P. The model can be more readily understood when comparing three conditions: [S] > Km. Match each statement with the condition that it describes. Note that "rate" refers to initial velocity Vo where steady state conditions are assumed. [Etotal] refers to the total enzyme concentration and [Efree] refers to the concentration of free enzyme. [S] > Km Almost all active sites will be filled. Adding more S will not increase the rate. Answer Bank Not true for any of these conditions Increasing [Etotal] will lower Km.arrow_forward(i) Which graph indicates an enzymatic reaction without inhibitor?(ii) Which type of inhibitor is it? Briefly explain.(iii) Which graph indicates the highest concentration of inhibitor?(iv) Calculate the Vmax and Km of the graph showing an enzymatic reaction with the lowest concentration of inhibitor. Show the steps of calculation and unit in your answers. Keep 2 decimal places in your answers.arrow_forwardYou begin to study enzyme Z, which catalyzes a simple reversible reaction that interconverts compound S and compound P. You observe that the ∆G´° for the S to P conversion to be –6 kJ/mol, and that compound S has ∆G´° for binding to enzyme Z of –15 kJ/mol, while compound P has a ∆G´° for binding to enzyme Z of –13 kJ/mol. Please explain the effect of enzyme Z on conversion of S to P. (Your answer should include a graph qualitatively showing energy versus reaction progress; however, you still need to explain youranswer in words!) not sure how to make the correct graph.arrow_forward
- A particular reaction has a ΔG‡ of 30.0 kJ mol-1 at 25.0 °C. In the presence of an enzyme, the same reaction has a ΔG‡ of 1.50 kJ mol-1 at the same temperature. Calculate the rate enhancement of this enzyme. (R = 8.3145 J mol-1 K-1)arrow_forwardThe Lineweaver-Burke plot was originally developed in order to "linearize" the data obtained from enzyme kinetics experiments, in order to facilitate the determination of kinetic parameters. Why is it not considered to be an accurate method for this purpose? It is very difficult to draw a straight line on a computer. It is very difficult to calculate the variables required for the "x" and "y" axis. It is more accurate to use the standard "V versus [S]" plot to determine Vmax and KM- The plot weights the least accurate data points the most heavily. It is no longer considered to be acceptable to extrapolate from known data.arrow_forwardHow can you find Kcat if you are only given Vmax, Km and [E] ? I tried using Kcat=Vmax/[E] but that didn't work. How do you know if [E] is the same as [E]total, and if it isn't how do you find it from this information: The Vmax for a particular enzyme is 10 nmols/L/s. The Km for its substrate is 5 microM. If the enzyme concentration is 10 nM, what is the kcat? a.80 nmoles/L/s b.8000 nmoles/L/s c.2 nmoles/L/s d.50 nmoles/L/sarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON