Question
Please show all work and circle your answer. Thank you
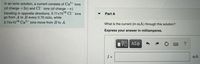
Transcribed Image Text:In an ionic solution, a current consists of Ca2+
(of charge +2e) and Cl ions (of charge -e)
traveling in opposite directions. 5.11x1018 Cl- ions
go from A to B every 0.70 min, while
3.74x1018 Ca²+ ions move from B to A.
ions
Part A
What is the current (in mA) through this solution?
Express your answer in milliamperes.
I =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
arrow_back_ios
arrow_forward_ios