
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
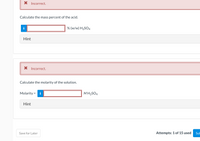
Transcribed Image Text:X Incorrect.
Calculate the mass percent of the acid.
% (w/w) H2SO4
Hint
X Incorrect.
Calculate the molarity of the solution.
Molarity = i
MH2SO4
%3D
Hint
Save for Later
Attempts: 1 of 15 used
Sub
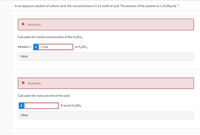
Transcribed Image Text:In an aqueous solution of sulfuric acid, the concentration is 9.11 mol% of acid. The density of the solution is 1.2528 g mL-1.
X Incorrect.
Calculate the molal concentration of the H2SO4.
Molality = i
0.908
m H2SO4
%3D
Hint
X Incorrect.
Calculate the mass percent of the acid.
i
% (w/w) H2SO4
Hint
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the Normality of Hcl of a solution that contains 45 g in 325 ml of water, knowing that the Molecular Mass of the Acid is 26.4 g / mol. Density is 1.19 g / ml and its purity is 27%.arrow_forwardHow many carbonate ions are present in 30.0 mL of 0.600 M K 2CO3 solution?arrow_forwardCalculate the normality of 0.321 g sodium carbonate when it mixes in a 250 mL solution.arrow_forward
- 2) Determine the molarity of a solution formed by mixing 8.56 g KIO; with sufficient water to obtain a total volume of 2000. mL.arrow_forward6.) Calculate the percent by mass of potassium sulfate in a solution made from 49.21 g potassium sulfate and347 mL of water. Assume the density of water is 0.976 g/mL.arrow_forwardplease put calculations in the given framearrow_forward
- What volume of 0.220 M HBr solution (in mL) is required to obtain 0.060 moles of HBr?arrow_forwarda) What mass of barium nitrate (Ba(NO3)2) would be required to prepare 2.000 L of a 0.0210 M aqueous solution of this salt? g(b) Calculate the mass percent of Ba(NO3)2 in this solution, assuming the density of the solution is 1.000 kg/L. %arrow_forwardA 10.4 g sample of an aqueous solution of perchloric acid contains an unknown amount of the acid. If 24.3 mL of 0.481 M potassium hydroxide are required to neutralize the perchloric acid, what is the percent by mass of perchloric acid in the mixture?arrow_forward
- What is the percent-by-mass concentration of acetic acid (CH3COOH) in a vinegar solution that contains 65.20 g acetic acid in a 1.000−L solution? The density of this solution is 1.005 g/mL.arrow_forward(a) How many milliliters of 0.145 M HCl are needed to neutralize completely 45.0 mL of 0.101 M Ba(OH)2 solution? 31.3 X ml (b) How many milliliters of 3.50 M H,SO4 are needed to neutralize 50.0 g of NaOH? 357.1 x mL (c) If 55.8 mL of BaCl, solution is needed to precipitate all the sulfate in a 544 mg sample of NazSO4 (forming BaSO4), what is the molarity of the solution? 0.047 X M (d) If 27.5 mL of 0.125 M HCI solution is needed to neutralize a solution of Ca(OH)2, how many grams of Ca(OH), must be in the solution? 0.25arrow_forwardA 12 fluid ounce serving of milk contains mostly water and about 413 mg of calcium. What is the concentration of calcium in the aqueous liquid in units of Part Per Million on a mass basis (ppmm). There are 33.814 fluid ounces per liter.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY