
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
in a titration, if the mass of KHP in three experimental solutions are 0.467,0.48, and 0.48 and the volume of NaOH IN THE exp are 22,29,24 respectively in order to the masses of KHP.find the molarity of NaOH of each experiment. calculate the avarage molarity of NaOH M.
Expert Solution
arrow_forward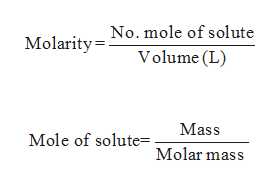

Step 1
Molarity: The concentration of the solutions is given by the term of molarity and it is given by ratio between numbers of moles of solute present in litter of solution.
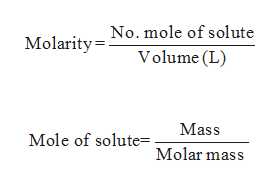
arrow_forward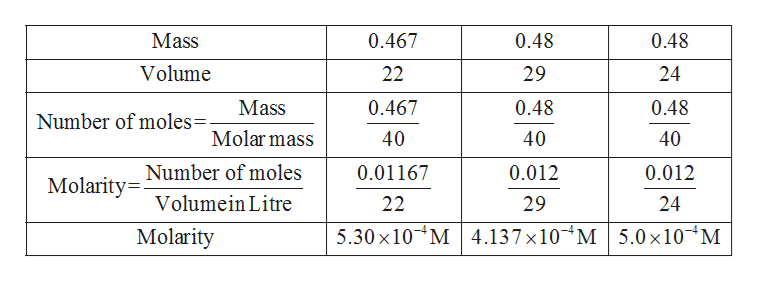

Step 2
Given data and calculation are given in table:

arrow_forward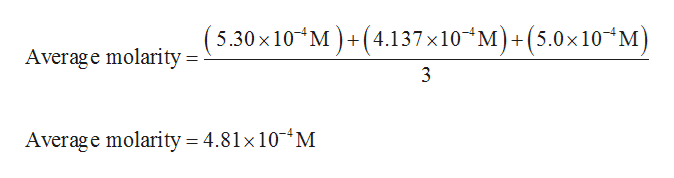

Step 3
Average molarity of NaOH solution is calculated as:
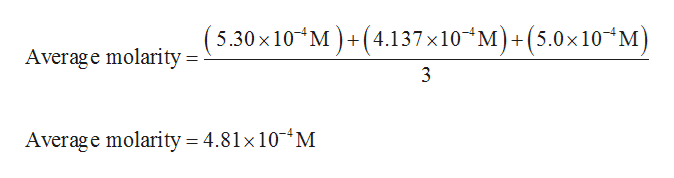
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In a titration, the equivalence point is the moment at which the number of moles of H+ from the acid that has been added is equal to the number of moles of OH- from the base that has been added. Remember that in a molecule like H2SO4, there are 2 moles of H+ per mole of H2SO4. 24.54 mL of 0.100M H2SO4(aq) is placed in a beaker. After 22.04 mL of NaOH is added to the beaker, the equivalence point is reached. What is the concentration (M) of the NaOH solution? Report your answer with 3 sig figsarrow_forwardA student is titrating a 100 mL of 0.50 M solution of hydrofluoric acid (HF) with 1.0 M strong base (NaOH). a) at the equivalence point, what ions/compounds will be present in the solution? A complete answer will also be specific as to what ions/compounds are not present in solution. b) calculate the pH at the equivalence point.arrow_forwardIn a titration, the equivalence point is the moment at which the number of moles of H+ from the acid that has been added is equal to the number of moles of OH- from the base that has been added. Remember that in a molecule like H2SO4, there are 2 moles of H+ per mole of H2SO4. 16.75 mL of 0.100M H2SO4(aq) is placed in a beaker. After 17.65 mL of NaOH is added to the beaker, the equivalence point is reached. What is the concentration (M) of the NaOH solution?arrow_forward
- 2. In the second titration, the molar mass of an unknown monoprotic acid is calculated. If 0.100 grams of unknown solid are dissolved in water and titrated with 4.68 mL of the NaOH, what is the molar mass of the solid? HINTS: Molar mass is grams / moles. We have grams from the weighed mass, we can calculate moles of acid from the titration. Monoprotic means that it produces 1 H+ per molecule. This means that our moles of NaOH added and moles of acid are the same. You will need to use your concentration of NaOH from the previous question Which is 0.1186arrow_forwardGW 18b 1. A 20.0 mL sample of a 0.240 M hydrofluoric acid (HF) solution is titrated with 0.200 M NaOH. Determine: (a) pH of the acid solution before any base is added; (b) volume (in mL) of base needed to get to the equivalence point; (c) pH halfway to equivalence point; (d) pH at equivalence point; (e) pH when 0.100 mL NaOH is added beyond the equivalence point. (K₂ of HF = 7.1 × 104)arrow_forwardIn this lab, we will experimentally determine the titration curve for the titration of a weak acid solution against a strong base solution. The weak acid we will use is potassium hydrogen phthalate, KHP, its chemical structure is shown below. KHP is an ionic compound composed of a potassium cation K+ and a hydrogen phthalate anion HP–. HP– is a weak acid and upon dissolving in water, can lower the pH of the solution. (a) Suggest the chemical reaction(s) when a solid sample of KHP is dissolved in water, writing out the chemical equations for them. (b) Sketch the structure of KHP from above and circle the hydrogen atom that is responsible for its acidity. (c) Calculate the pH of a solution made of 0.50 g of KHP and 50 mL of water. KHP has a molar mass of 204.2 g and at 25 °C has a pKa of 5.4.arrow_forward
- 4. What is the pH at each of the following points in the titration of 25.00 mL of 0.100 M CH,CH;COOH with 0.100 M NaOH? a) before the addition of any NaOH; b) after the addition of 10.00 mL of 0.100 M NaOH; c) after the addition of 12.50 mL of 0.100 M NaOH; d) after the addition of 25.00 mL of 0.100 M NaOH; e) after the addition of 26.00 mL of 0.100 M NaOH.arrow_forwardAfter the benzoate ion is separated from methyl benzoate because of their differences in solubility, what happens when HCI is added to the benzoate ion dissolved in water? OHCI donates a proton to the benzoate ion, resulting in benzoic acid, which is not soluble in water HCI accepts a proton from the benzoate ion, resulting in benzoic acid, which is not soluble in water HCI donates a proton to the benzoate ion, resulting in benzoic acid, which is soluble in water OHCI accepts a proton from the benzoate ion, resulting in benzoic acid, which is soluble in waterarrow_forwardCalculate the pH for each of the cases in the titration of 35.0 mL of 0.240 M KOH(aq) with 0.240 M HCl(aq). Note: Enter your answers with two decimal places. before addition of any HCl: after addition of 13.5 mL HCl: after addition of 21.5 mL HCl: after addition of 35.0 mL HCl: after addition of 41.5 mL HCl: after addition of 50.0 mL HCl:arrow_forward
- Write the titration reaction of 100 mL of 0.100 M anilinium bromide (“aminobenzene . HBr”) +.100 M NaOH.arrow_forwardA student performs the titration of 88.0 mL of 1.1 M HCI (in flask) with 13.0 mL of 0.20 M NaOH (in burette). What is the pH at this point in the titration? (Hint: Use a barf table.) Your Answer: Answerarrow_forward5. You use a 0.1380 M HCI to standardize an NaOH solution. The burets below show initial and final volume readings for the titration to the perfect endpoint. Calculate the appropriate volume ratio (Va/Vb or Vb/Va ratio) and the [NaOH]. Acid Buret Reading (mL) initial final cumpasın INHI IN ||| Base Buret Reading (mL) initial final ייייייי |||||arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY