
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
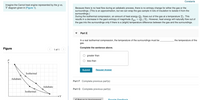
Transcribed Image Text:Constants
Imagine the Carnot heat engine represented by the p vs.
V diagram given in (Figure 1).
Because there is no heat flow during an adiabatic process, there is no entropy change for either the gas or the
surroundings. (This is an approximation, but we can wrap the gas sample in lots of insulation to isolate it from the
surroundings.)
During the isothermal compression, an amount of heat energy Qc flows out of the gas at a temperature Tc. This
results in a decrease in the gas's entropy of magnitude S = Qc/Tc. However, heat energy will naturally flow out of
the gas into the surroundings only if there is a (slight) temperature difference between the gas and the surroundings.
gas
Part E
In a real isothermal compression, the temperature of the surroundings must be
the temperature of the
gas.
Figure
Complete the sentence above.
1 of 1
>
greater than
less than
Submit
Request Answer
Isothermal
Adiabatic
Part F Complete previous part(s)
Adiabatic
Part G Complete previous part(s)
Isothermal
→V
( Return to Assianment
Provide Eeedback
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A piston-cylinder with 25 grams of saturated water within. Is kept at a pressure of 300 kpa. A heater inside supplies We,in =7.2kJ while the cylinder loses 3.7 kJ of heat. Show that the boundary Wb and the change in internal energy ∆U in this problem can be combined to form ∆H anf find the final temperatire in Celsius.arrow_forwardI need help on this question: This question requires the use of Thermodynamics Property Tables. In addition, for steam, the specific ideal gas constant = 461.5 J/kg K. Q. A closed system is comprised of 10 kg pure water substance initally at a temperature of 400 oC and a pressure of 8.5 MPa (State 1). a) For state 1, show that the system is in the superheated vapour phase. b) Evaluate the specific volume of the steam in state 1 using the thermodynamic property tables supplied and compare it to the value obtained assuming the steam behaves as an ideal gas. Which value woyld you have most confidence in (give reasons for your choice).arrow_forwardI need help with this. Thermodynamicsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY