
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Q No 62 Kindly solve this question correctly in 30 minutes and get the thumbs up please show me neat and clean work for
Kindly provide correct solution
Transcribed Image Text:9
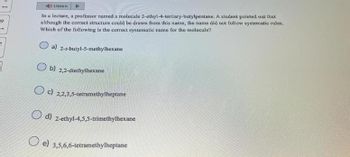
In a lecture, a professor named a molecule 2-ethyl-4-tertiary-butylpentane. A student pointed out that
although the correct structure could be drawn from this name, the name did not follow systematic rules.
Which of the following is the correct systematic name for the molecule?
a) 2-1-butyl-5-methylhexane
b) 2,2-diethylhexane
O c 2,2,3,5-tetramethylheptane
Listen
d) 2-ethyl-4,5,5-trimethylhexane
e) 3,5,6,6-tetramethylheptane
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Thanks in advance!arrow_forwardSaved Normal BIIIU fxl Ix Actual mass used in solution prep (g) Volume solution prepared Measured conductivity (uS/cm) Substance 0.1 g Naci 0.1003 100 mL 1149 0.1 g Nal 0.10 100 ml 419 Calculate the mass of Nal that would be necessary to yield the same conductivity as the NaCl solution. Clearly show these calculations in your lab notebook. Saved T BII U X X + fr Normalarrow_forwardPlease show work, just like I need to put it in. Make clear HOW you got to that answer. Please write neat & make it readable. Again please write like I would need to put it in!arrow_forward
- DATA AND CALCULATIONS Show all calculations neatly on an attached sheet. Trial 1 Trial 2 Trial 3 70.6849. 68.0749 2.61g 23.25ML O.65ML 22.6mL Mass of flask + vinegar Mass of empty flask Mass of vinegar used Final buret reading Initial buret reading Volume of NaOH used Moles of NaOH used 0.00226 mol Moles of açetic acid titrated Mass of acetic acid titrated Mass % acetic acid in vinegar 5.0%arrow_forwardHello, I hope you are doing well on this fine day. For the following quetion please read carefully the question and instruction. PLEASE ANSWER QUESTION IN 20 MINTUES NOT MORE PLEASE AND THANK YOU. If you do answer the question correctly and post it in the next 15 minutes, NO NEED TO SHOW THE WORK, I JUST WOULD LIKE THE CORRECT ANSWER AS SOON AS POSSIBLE. I will write a wonderful and generous feedback/review/rating about you. 2.077 g of salicylic acid is used in the reaction to synthesize ethyl salicylate, what is the minimum amount of ethanol in mL necessary to convert all of the salicylic acid to ethyl salicylate? The molecular weight of salicylic acid is 138.12 g/mol, the molecular weight of ethyl salicylate is 166.176 g/mol. The molecular weight of ethanol is 46.069 g/mol and its density is 0.789 g/mL.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY