
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
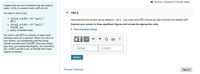
Transcribed Image Text:I Review I Constants I Periodic Table
Imagine that you are in chemistry lab and need to
make 1.00 L of a solution with a pH of 2.40.
You have in front of you
Part A
• 100 mL of 6.00 x 10-'mol L-1
HCI,
• 100 mL of 5.00 x 10-2mol L-1
NaOH, and
• plenty of distilled water.
Assuming the final solution will be diluted to 1.00L, how much more HCl should you add to achieve the desired pH?
Express your answer to three significant figures and include the appropriate units.
• View Available Hint(s)
You start to add HCl to a beaker of water when
someone asks you a question. When you return to
HA
?
your dilution, you accidentally grab the wrong
cylinder and add some NaOH. Once you realize
your error, you assess the situation. You have 85.0
mL of HCl and 90.0 mL of NaOH left in their
original containers.
Value
Units
Submit
Provide Feedback
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A solution contains 0.48 mol of HF (pKa = 3.75). 0.24 mol of NaOH are added, and the solution is brought to a final volume of 2 L. Without using a calculator, what is the pH of the solution? A) pH is 3.75 B) pH is less than 3.75 C) pH is greater than 3.75 D) pH is less than 10.25 E) pH is 7.0arrow_forwardThe ___________ of the buffer system in human blood is present in excess. weak acid conjugate basearrow_forwardCalculate the pH after 0.20 mol KOH is added to 1.00 L of the solution 7.2. The solution is 7.2. Calculate the pH after 0.20 mol KOH is added to 1.00 L of that solution.arrow_forward
- Lakes that have been acidified by acid rain can beneutralized by liming, the addition of limestone(CaC03)arrow_forwardCalculate the pH of the glacial acetic acid and the solution you prepared in step 1. Step 1: Glacial acetic acid, the most concentrated CH3COOH you can buy, has a concentration of 17.54 M. Calculate the concentration of CH3COOH is a solution that is prepared by combining 15.0 mL of glacial acetic acid with enough water to make 250-mL of solution. (Please be very detailed when answering the question)arrow_forwardThe pH after adding 21.0 mL of 1.0 M NaOH to 25.0 mL of 1.0 M HCl is 0.087 0.16 0.80 1.1arrow_forward
- Would the concentration of hydroxide ion in a 0.20 M solution of sodium hydroxide (NaOH) be greater than, less than, or equal to 0.20 M?arrow_forwardA chemist makes a new solution: 1.0L of a solution that is both that is both 0.6 M NH; and 0.8 M NH,CI. What is the pH of the solution after 0.10 mol of NAOH is added? Round your answer to 2 decimal places. There is no change in volume upon addition of NaOH. pH =arrow_forwardplease solve both question. each image has different question.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY