Question
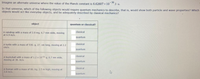
Transcribed Image Text:Imagine an alternate universe where the value of the Planck constant is 6.62607 x 10
36
J-s.
In that universe, which of the following objects would require quantum mechanics to describe, that is, would show both particle and wave properties? Which
objects would act like everyday objects, and be adequately described by classical mechanics?
object
quantum or classical?
classical
A raindrop with a mass of 2.0 mg, 6.7 mm wide, moving
at 6.9 m/s.
quantum
A turtle with a mass of 530. g, 27. cm long, moving at 2.2
classical
cm/s.
quantum
classical
A buckyball with a mass of 1.2 x 1021 g, 0.7 nm wide,
moving at 38. m/s.
quantum
classical
A human with a mass of 86. kg, 2.5 m high, moving at
3.0 m/s.
quantum
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- 1. If it moves at a speed of 900 m/s, what is the wavelength (a) electrons; (b) 25000 kg aircraft; (c) Which of the two acts as a matter wave? explain! 2. Green light has a wavelength of 550 nm, through what potential difference must the electrons be accelerated to have a wavelength like this?arrow_forwardi know the answer is NOT 9.42(10^-21)arrow_forwardThe allowed energies of a quantum system are 0.0 eV, 5.5 eV , and 9.0 eV. What wavelengths appear in the system's emission spectrum? (Express your answers in nanometers in ascending order separated by commas).arrow_forward
- In an experiment to study the photo-electric effect, light of wavelength 400 nm shining on a metallic surface causes electrons to be emitted. The maximum kinetic energy of the electrons is 1.05 eV (electron volts). Another light source of wavelength 200 nm is then tried, and the emitted electrons have a maximum kinetic energy of 4.20 eV. Using this information: a) determine the value of Planck's constant. b) determine the work function of the metal.arrow_forwardElectrons striking the back of a conventional TV screen travel at a speed of about 2.7 ✕ 107 m/s. What is their de Broglie wavelength (in nm)?arrow_forwardThe diameter of a Hydrogen atom is about 10.6 nm. What is the minimum uncertainty in momentum of an electron along this diameter if it's uncertainty in position is equal to the diameter of the atom? a) 1.17 x 10^-25 kgm/s b)9.94 x 10^-26 kgm/s c)1.17x10^-26 kgm/s d) 9.94x10^-27 kgm/s e)arrow_forward
- An elementary particle has a very short lifetime, 1.34 x 101 s. What is the minimum uncertainty with which we can measure its energy? Please give your answer in electron Volts.arrow_forwardA particle of matter is moving with a kinetic energy of 7.53 eV. Its de Broglie wavelength is 2.85 x 10^-12 m. What is the mass of the particle?arrow_forward= 1. Photoelectric effect. In a photoelectric experiment in which monochromatic light of wave- length \ falls on a potassium surface, it is found that the stopping potential Vstop is 1.9 V for > 300 nm and 0.88 V for \ = 400 nm. Imagine we know neither Planck's constant, nor the workfunction for potassium, nor the threshold frequency fo. But assume we do know the elementary charge e 1.60 × 10-19 C and want to test the theoretical prediction of Eintsein's theory. = (a) From the given data, calculate a value for Planck's constant, h. (b) From the same data, find the workfunction Eo and the threshold frequency fo for potas- sium. (c) Then compare your results for h and Eo to their known values (see Knight, Table 38.1 for the work function). (d) Plot eVstop as a linear function of frequency f. Include the information you have found in parts (a) and (b) as well as the experimental data.arrow_forward
arrow_back_ios
arrow_forward_ios