
Concept explainers
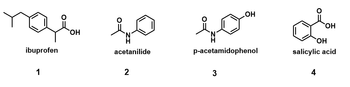
Lily has a mixture of 4 compounds that they want to separate. All 4 compounds appear as white solid powders. When Lily adds 25 mL of chloroform (density = 1.492 g/mL; bp = 61C) to the mixture, everything dissolves with stirring. Lily decides to use acid-base extraction to separate the compounds from one another. Chloroform and water are immiscible, and all acids and bases will be prepared in water.
1. First, Lily tries extracting the organic solution with 1.0 M HCl (20 mL). Which layer will be the organic layer in the separatory funnel?
5. Calculate the pH of the aqueous combined aqueous layers. Remember that pH = - log[H+] where the concentration is in M.
6. After combining the two aqueous layers and adjusting the pH to 2, Lily observes solid precipitate out of solution. They cool it in an ice bath and then collect the solid by vacuum filtration. What is this solid?
- 1,4 as salts
- 1,3,4 as salts
-1,4
-1,3,4
7. Lily realizes that the solid they have collected is not a pure compound but a mixture. They re-dissolve it in 25 mL of chloroform and try extracting with 5% NaHCO3 (20 mL) instead. What will be in the aqueous layer after this step?
- Re-dissolve in chloroform and extract with HCl
- Re-dissolve in chloroform and extract with NaHCO3
- They cannot be separated from each other with acid-base extraction
- Re-dissolve in chloroform and extract with NaOH
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Will anything be left in this new organic layer (from Q20)?
Will anything be left in this new organic layer (from Q20)?
nothing will be in the organic layer
3
2
3 as a salt
Will anything be left in this new organic layer (from Q20)?
Will anything be left in this new organic layer (from Q20)?
nothing will be in the organic layer
3
2
3 as a salt
- it is time for the titration. You measure 15.0 mL of the H2C2O4 solution with a 100-mL graduated cylinder and add it to a 125-mL Erlenmeyer flask. Then you add two drops of the indicator, phenolphthalein, to the oxalic acid solution in the flask. You dispense the NaOH solution from the buret into the flask (while swirling the flask) until you reach the endpoint. At the endpoint, the solution in the flask turns light pink. You repeat the titration two additional times for a total of three trials. H2C2O4 (aq) + 2 NaOH (aq) --> Na2C2O4 (aq) + 2 H2O (l) (I) (II) (III) Volume of H2C2O4 used 15.00 mL 15.00 mL 15.00 mL Initial Buret Reading 0.00 mL 0.05 mL 0.10 mL Final Buret Reading 14.45 mL 14.38 mL 15.40 mL Volume NaOH used ________mL ________mL ______mL Moles NaOH _______M ________M _________M…arrow_forwardA chemistry student weighs out 0.159 g of ascorbic acid (H,C,H,0), a diprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.0800M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Be sure your answer has the correct number of significant digits. mLarrow_forwardWhat do you understand about the manufacture of chemical solutions? How to (a) make 1000 mL of 0.2 N hydrochloric acid solution from 1 N standard solution and (b) make 100 mL of 0.5 N sodium hydroxide secondary standard solution (known BM = 40 g / mL)? Explain!arrow_forward
- The solubility product constant for a certain metal fluoride, MF2 is Ksp = 1.2 × 10−6. Its molar mass is ℳ = 175.90 g/mol. What is its solubility in mol/L? Report your answer to TWO significant figures. Enter your answer in scientific notation using the appropriate boxes. Remember, a number like 1.6, in scientific notation is 1.6 × 100. Note: Your answer is assumed to be reduced to the highest power possible.arrow_forwardThe total cation content of natural water is often determined by exchanging the cations for hydrogen ions on a strong acid ion-exchange resin. A 25.00 mL sample of a natural water was diluted to 100.00 ML with distilled water, and 2.06 g of a cation - exchange resin was added. After stirring, the mixture was filtered and the solid remaining on the filter paper was washed with three 15.00 mL portions of water. The filtrate and washings required 16.30 mL of 0.0282 M NaOH to give a bromocresol green end point. a) Calculate the number of millimoles of cation present in exactly 1.00 L of sample. b ) Report the results in terms of milligrams of CaCO3 per liter. Only typed solution.arrow_forwardA chemist needs to determine the concentration of a sulfuric acid solution by titration with a standard sodium hydroxide solution. He has a 0.1886 M0.1886 M standard sodium hydroxide solution. He takes a 25.00 mL25.00 mL sample of the original acid solution and dilutes it to 250.0 mL.250.0 mL. Then, he takes a 10.00 mL10.00 mL sample of the dilute acid solution and titrates it with the standard solution. The endpoint was reached after the addition of 12.42 mL12.42 mL of the standard solution. What is the concentration of the original sulfuric acid solution?arrow_forward
- 4. A student did not read the directions to the experiment properly and mixed up where to put the NaOH and the HCl solutions. He put the HCl in the buret and the NaOH in the flask. He then added a drop of the phenolphthalein to the solution in the flask. Does the student need to empty out all of the solutions and start over again or can he go ahead and run the titration? Explain qalb ribidw qid edini olddud ris ogrel s znisimos tund entd nousu 5. How many liters of 3.4 M HI will be required to reach the equivalence point with 2.1 L of 2.0 M KOH? 9no vino to beateni nolusius to alsid sigulum ob of insttoqmi ti al VW Sarrow_forwardPerform the following calculations for your procedure: 1. To prepare a 100.0-mL 4.00 mM Fe³+ solution, how much solid of FeCl, would you need to weigh on the balance? 2. Fill in the following table for your diluted FeCl, standard solutions to determine the volume of the 4.00 mM stock solution you'd need to prepare 1.50-mL of each of the following dilutions. You may choose 4 concentrations between 0 and 4.00 mm. [Fe3+] (mM) Stock vol (μl) 4.00 0.00arrow_forwardAn analytical chemist weighs out 0.050 g of an unknown triprotic acid into a 250 mL volumetric flask and dilutes to the mark with distilled water. He then titrates this solution with 0.1600 M NaOH solution. When the titration reaches the equivalence point, the chemist finds he has added 9.6 mL of NaOH solution. Calculate the molar mass of the unknown acid. Be sure your answer has the correct number of significant digits. g molarrow_forward
- An analytical chemist weighs out 0.185 g of an unknown monoprotic acid into a 250 mL volumetric flask and dilutes to the mark with distilled water. She then titrates this solution with 0.2000 M NAOH solution. When the titration reaches the equivalence point, the chemist finds she has added 9.5 mL of NaOH solution. Calculate the molar mass of the unknown acid. Be sure your answer has the correct number of significant digits. g x10 molarrow_forwardCalculate the molar mass of the unknown acid. Be sure your answer has the correct number of significant digits.arrow_forwardA chemistry student needs to standardize a fresh solution of sodium hydroxide. She carefully weighs out 51 mg of oxalic acid (H2C2O4), a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in 250 mL of distilled water. The student then titrates the oxalic acid solution with her sodium hydroxide solution. When the titration reaches the equivalence point, the student finds she has used 19.2 mL of sodium hydroxide solution. Calculate the molarity of the student's sodium hydroxide solution.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





