Question
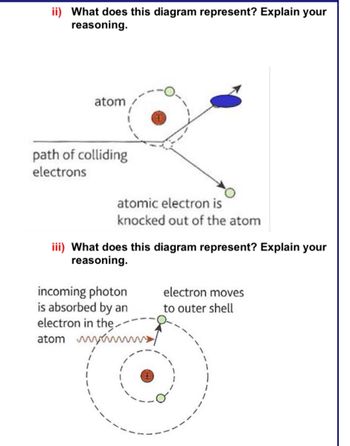
Transcribed Image Text:ii) What does this diagram represent? Explain your
reasoning.
atom
path of colliding
electrons
atomic electron is
knocked out of the atom
iii) What does this diagram represent? Explain your
reasoning.
incoming photon
is absorbed by an
electron in the
atom wwwww
electron moves
to outer shell
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Solve all problemsarrow_forward25. According to the image below answering all the questions. Each transition is labeled with a letter (A,B, etc) near its arrowhead. Each transition can be radiative, non-radiative, or both. (A) Select the arrow(s) that correspond to electronic excitation. (B) Select the arrow(s) that correspond to vibrational excitation. (C) Select the arrow(s) that correspond to IR absorption. (D) Select the arrow(s) that correspond to UV-visible absorption. (E) Select the arrow(s) that correspond to emission of a UV-visible photon. (F) Select the arrow(s) that correspond to thermal relaxation (non-radiative). (G) Select the arrow(s) that correspond to a chemical reaction (e.g. fragmentation). (H) Select the arrow(s) that correspond to intersystem crossing. All choosing options are the same as indicated below. -A -B -C -D -F -G -Harrow_forwardQUESTION 8 An electron in hydrogen absorbs a photon and jumps from orbit n = 2 to n = 4. Using the energy level diagram shown, what was the energy of the absorbed photon? a. 0 eV b. 0.85 eV c. 3.4 eV d. 10.2 eV e. 2.55 eVarrow_forward
- Question 9 The HVL for lead atomic number 82 of attenuation 0.1 is 69.3 6.930 0.693 0.0693arrow_forward3. Moder physics question Please provide a correct, readable, and well explained solution for the followingarrow_forward4. The energy of the atom is in part determined by the electrical potential energy of the electron interacting with the proton nucleus, and when the electron is close to the nucleus it is very tightly bound. That's why the energy is negative, and larger in magnitude the smaller n. What does this tell you about the size of the atom for large n? Explain your reasoning.arrow_forward
arrow_back_ios
arrow_forward_ios