
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
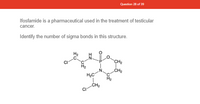
Transcribed Image Text:**Question 28 of 39**
Ifosfamide is a pharmaceutical used in the treatment of testicular cancer.
Identify the number of sigma bonds in this structure.
**Chemical Structure Description:**
The image shows the structural formula of ifosfamide. It includes:
- A phosphorus atom (P) with a double bond to an oxygen atom (O) and two single bonds to nitrogen atoms (N).
- The phosphorus atom is also connected to an oxygen atom in a ring structure.
- There are several carbon (C) and hydrogen (H) atom chains attached to nitrogen atoms, forming parts of the molecular structure.
- Two chlorine atoms (Cl) are bonded to carbon atoms in the structure.
**Task:**
Count the number of sigma bonds in the depicted chemical structure.
**Note:**
- Sigma bonds represent single covalent bonds formed between two atoms.
- In a molecular structure, each line or single bond represents one sigma bond.
- To determine the total number of sigma bonds, add up all the single lines connecting the atoms.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Rotation about which bond changes the dihedral angle designated ? N-Ca C. C C R C-Narrow_forwardDraw the 2D and the 3D Lewis structure for each molecule. You do not need to show resonance structures here. Determine the electron pair geometry (EPG), the molecular geometry (MG), the bond angle (BA) around the central atom, and the hybridization of the central or bold atom. Circle the formula of any polar molecule. For molecules with more than one central atom (e.g. ??3??2??) give the structure around the bold atom.arrow_forwardhalf of t bond Which of the following is an accurate representation of bonding in ethylene (H2C=CH2)? A B H. half of T bond sp HA C pOsp C H. H H. sP H. sp ethylene o bond framework (viewed from above the plane) a bond (viewed from alongside the plane) O C only all three B only O A only A and Barrow_forward
- Fill in any nonbonding valence electrons that are missing from the following structures.arrow_forwardThe amount of UVA radiation hitting a surface at sea level in a lightly clouded day is about 70W/m2 . About half of that can be absorbed by the skin. A typical carboncarbon bond requires 348 kJ/mol to break. A person lies on the beach for about 1 hour without sunscreen (i.e. fully exposed to UVA radiation). Estimate the number of C-C bonds broken in this person’s back (about 0.18 m2 ) over that period. Assume that the average wavelength of UVA is 335 nm.arrow_forwardBiotin is being studied in a lab. a. What is the IMF(s) of biotin (C10H16N2O3S)? b. What are the sigma/pi bondings and bond angles of biotin? c. What are the resonance functional groups?arrow_forward
- Please answer the following questions about the below molecule: This molecule has ___ sigma bonds. This molecule has ___ pi bonds. This molecule has ___ hydrogen atoms. This molecule has ___ carbon atoms. This molecule has ___ lone pairs The nitrogen atom is ___ hybridized. The ___ orbital is involved in all hydrogen sigma bonding. The bond angle of C-N-O is ___ °.arrow_forwardWhich molecules are polar? For each that is polar, specify the direction of its dipole moment. Q.HCNarrow_forwardNumber of Molecule valence electrons Formal Charge Electron-Group Geometry Molecular Geometry Resonance COSe C: C: Select one ... C: Select one.. Select one .. SH412 S: S: Select one .. S: Select one .. Select one ... SeHĄBr2 Se: Se: Select one... Se: Select one ... Select one ... AtGeN Ge: Ge: Select one ... Ge: Select one ... Select one ... CIGEP Ge: Ge: Select one... Ge: Select one ... Select one ... V 10000arrow_forward
- Number of Molecule valence electrons Formal Charge Electron-Group Geometry Molecular Geometry Resonance HỌCI 14 O: 2- 0: Select one ... O: Select one ... Select one .. GEHATCII Ge: Ge: Select one ... Ge: Select one ... Select one SAts S: S: Select one ... S: Select one ... Select one . SbAt2Cl2* Sb: Sb: Select one .. Sb: Select one ... Select one ... PCI4* P: P: Select one ... P: Select one .. Select one ..arrow_forwardWhich are the correct orbital overlaps to describe the circled bonds? 2 One sp²-sp² π (pi) overlap and one 2p-2p π (pi) overlap Two sp²-sp² a (sigma) overlaps One sp²-sp² a (sigma) overlap and one 2p-2pm (pi) overlap Two 2p-2p п (pi) overlaps One sp²-sp² o (sigma) overlap and one 3p-3p π (pi) overlaparrow_forwardFill in the appropriate information. How do I fill each one?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY