
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
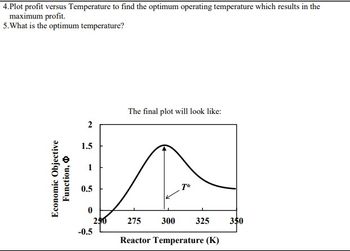
Transcribed Image Text:4.Plot profit versus Temperature to find the optimum operating temperature which results in the
maximum profit.
5. What is the optimum temperature?
Economic Objective
Function,
1.5
1
0.5
0
250
-0.5
The final plot will look like:
T*
275 300 325
Reactor Temperature (K)
350
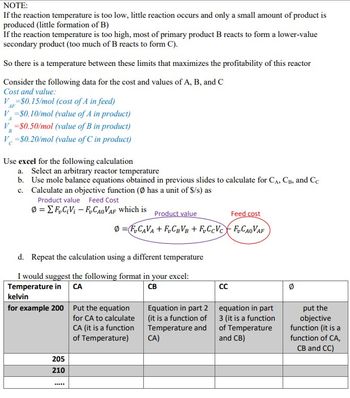
Transcribed Image Text:NOTE:
If the reaction temperature is too low, little reaction occurs and only a small amount of product is
produced (little formation of B)
If the reaction temperature is too high, most of primary product B reacts to form a lower-value
secondary product (too much of B reacts to form C).
So there is a temperature between these limits that maximizes the profitability of this reactor
Consider the following data for the cost and values of A, B, and C
Cost and value:
V=$0.15/mol (cost of A in feed)
AF
V=$0.10/mol (value of A in product)
V=$0.50/mol (value of B in product)
V=$0.20/mol (value of C in product)
A
B
Use excel for the following calculation
a. Select an arbitrary reactor temperature
b. Use mole balance equations obtained in previous slides to calculate for CA, CB, and Cc
c. Calculate an objective function (Ø has a unit of $/s) as
Product value Feed Cost
Ø-EF₂C₁V₁-F₂CAOVAF which is
d. Repeat the calculation using a different temperature
I would suggest the following format in your excel:
Temperature in CA
CB
kelvin
for example 200
205
210
Product value
Feed cost
@ =FuCV+FCBVB+FCcVct FyCaoVAF
***
Put the equation
for CA to calculate
CA (it is a function
of Temperature)
Equation in part 2
(it is a function of
Temperature and
CA)
CC
equation in part
3 (it is a function
of Temperature
and CB)
put the
objective
function (it is a
function of CA,
CB and CC)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- If 5.9 kg of CO, are produced during a combustion reaction, how many molecules of CO, would be produced?arrow_forwardThe point in the phase diagram where the fusion curve, the vapor pressure curve, and the sublimation curve join is called the Question 4 options: melting point. critical point. double point. boiling point. triple point. A sample of an ideal gas is slowly compressed to one-half its original volume with no change in temperature. What happens to the average speed of the molecules in the sample? Question 5 options: It doubles. It halves. cannot be determined without more information. It quadruples. It does not change. Phase changes occur Question 6 options: as the temperature decreases. all of the above. none of the above. as the temperature remains the same. as…arrow_forwardTake substantial derivative of desity equation ro = (t^(2 / 3)) + (x ^ 2) - y at (x, y, t) = (1, 1, 1)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY