
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:If the pH is 2, what is the %HA at of a molecule has a pka = 6?
Report the answer to one decimal place.
Your Answer:
Answer
units
Expert Solution
arrow_forward
Step 1
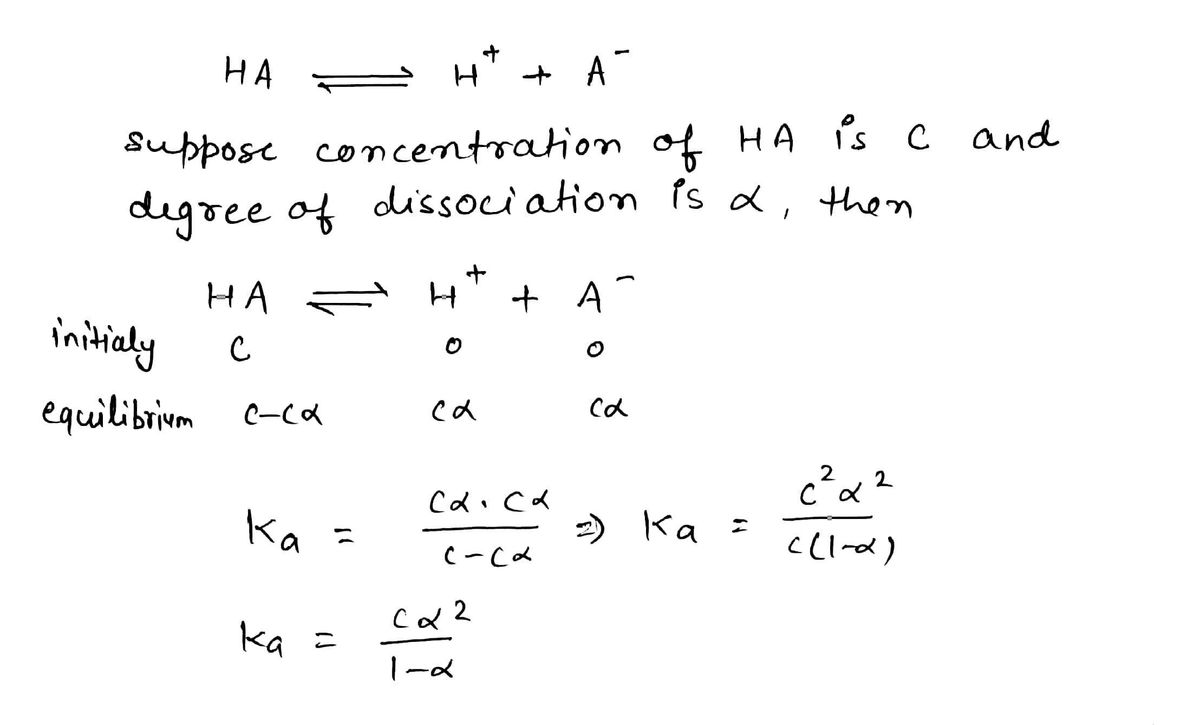
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemistry student weighs out .119g of hypobromous acid (HBrP) into a 250 mL volumetric flask and diluted to the mark with distilled water, he plans to titrate the acid with 0.0600M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the equivalence point.arrow_forwardIf the pKa of a molecule is 3, what is the %A- at pH 67 Report the answer to one decimal place. Your Answer: Answer units Amino Acid pKac pKan pKagarrow_forwardWhat is the molarity of a solution containing 12.7 g of sodium carbonate in 1.5 Lof solution? Round your answer to the thousandths place and do not include units.arrow_forward
- How would I calculate the amount of each component in each solution? Stock solutions provided: 0.5 M EDTA, pH 8 10% bromophenol blue 10% xylene cyanol Sucrose powder Tris powder Glacial acetic acid Solution A: 50 mL of 50x Tris Acetate EDTA (TAE) Buffer 2M Tris Base 50mM EDTA, pH 8 11.4% (v/v) glacial acetic acid Solution B: 1L of 1x TAE Buffer 40mM Tris-acetate 1mM EDTA Solution C: 5mL of 10x Loading Dye 0.25% (w/v) bromophenol blue 0.25% (w/v) xylene cyanol 66.5% (w/v) sucrose 100mM EDTA, pH 8arrow_forward(please type answer).arrow_forwardIn 300-400 words (which is about 20 sentences, 1 longer paragraph, or 2 shorter paragraphs), please use your own words to summarize the chemical properties of water and explain two or more reasons why living things need water.arrow_forward
- 5arrow_forwardIf there is an increase in the hydrogen ion concentration from 38 nmol.l-1 to 82 nmol.l-1, what does the pH change from and to?arrow_forwardThe pH scale shows availability of reactive hydrogen ions (H+) in a liquid. The scale is logarithmic, so milk has 10 times as many H+ ions as pure water, for a given volume. How many more H+ ions does soda pop have compared to pure water? Write the number only.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON