
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
![**Transcription for Educational Website:**
---
**Heat of Combustion Calculation**
In this exercise, you are given the following problem:
"If the heat of combustion for a specific compound is –1120.0 kJ/mol and its molar mass is 98.67 g/mol, how many grams of this compound must you burn to release 872.10 kJ of heat?"
**Calculation Requirements:**
- **Heat of Combustion**: –1120.0 kJ/mol
- **Molar Mass**: 98.67 g/mol
- **Target Energy Release**: 872.10 kJ
**Solution Steps:**
1. **Determine the Number of Moles Needed**:
- Use the equation:
\[
\text{moles} = \frac{\text{Target Energy Release}}{\text{Heat of Combustion}}
\]
2. **Calculate the Mass**:
- Multiply the number of moles by the molar mass to find the mass required.
**Mass Required**:
\[ \text{mass} = \]
---
*Please input the calculations in the box provided to find the precise mass in grams.*](https://content.bartleby.com/qna-images/question/76884ef0-0050-4a4d-a83f-5c6d84fc3fcd/faf0d1ca-e06e-485e-8b2f-2995da3eaca6/jkp6wu_thumbnail.jpeg)
Transcribed Image Text:**Transcription for Educational Website:**
---
**Heat of Combustion Calculation**
In this exercise, you are given the following problem:
"If the heat of combustion for a specific compound is –1120.0 kJ/mol and its molar mass is 98.67 g/mol, how many grams of this compound must you burn to release 872.10 kJ of heat?"
**Calculation Requirements:**
- **Heat of Combustion**: –1120.0 kJ/mol
- **Molar Mass**: 98.67 g/mol
- **Target Energy Release**: 872.10 kJ
**Solution Steps:**
1. **Determine the Number of Moles Needed**:
- Use the equation:
\[
\text{moles} = \frac{\text{Target Energy Release}}{\text{Heat of Combustion}}
\]
2. **Calculate the Mass**:
- Multiply the number of moles by the molar mass to find the mass required.
**Mass Required**:
\[ \text{mass} = \]
---
*Please input the calculations in the box provided to find the precise mass in grams.*
Expert Solution
arrow_forward
Step 1
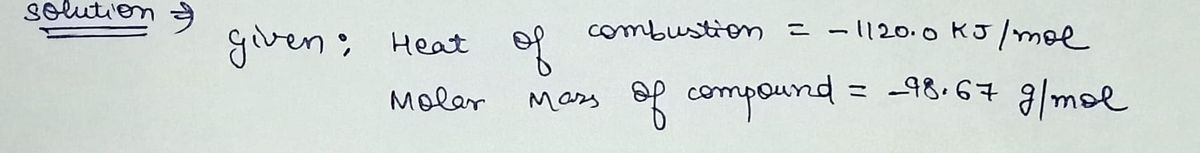
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hydrogen sulfide, H2 S, is produced during decomposition of organic matter. When 0.4300 mol H2S burns to produce SO2 (g) and H20(g), -222.7 kJ of heat is released. What is this heat in kilocalories? Heat kcalarrow_forwardCalculate the amount of heat released from combustion of 1 g of woodarrow_forwardIf the heat of combustion for a specific compound is-1440.0 kJ/mol and its molar mass is 43.65 g/mol, how many grams of this compound must you burn to release 465.70 kJ of heat? Numberarrow_forward
- Hydrogen sulfide is produced during the decomposition of organic matter. When 0.5380 mol H2S burns to produce SO2 (g) and H2O (g) , -278.7 kJ of heat is released. What is this heat in kilocalories?arrow_forwardA certain chemical reaction releases 33.8/kJg of heat for each gram of reactant consumed. How can you calculate what mass of reactant will produce 810.J of heat? Set the math up. But don't do any of it. Just leave your answer as a math expression. Also, be sure your answer includes all the correct unit symbols.arrow_forwardIf the heat of combustion for a specific compound is -1160.0 kJ/mol and its molar mass is 92.31 g/mol, how many grams of this compound must burn to release 834.70 kJ of heat? you mass:arrow_forward
- A metal object with a mass of 28.6 g is heated to 97.0 C and then transferred to an insulated container containing 91.2 g of water at 20.5 C. The water temperature rises and the temperature of the metal object falls until they both reach the same final temperature of 25.0 C. What is the specific heat of this metal object? Assume that all the heat lost by the mental object is absorbed by the water. specific heat: ? cal/ g.Carrow_forward#3arrow_forward4. Determine the final temperature of the solution formed when 0.31 g sodium oxide is added to 100 ml water in a calorimeter. Assume that the volume of the resulting solution is 100 ml. The temperature before reaction is 19°C. The heat capacity of the calorimeter is 20J/°C. Suppose that the specific heat and density of the solution are the same as those of water. Write down all the reaction equations needed to solve this exercise.arrow_forward
- Calculate the change in energy for the following processes and identify if the process is endothermic or exothermic. 1. How much heat is released by a system when 45 J of work is done on it to decrease its energy to 23 J? 2. What is the work done by a system when it absorbs 47 J of heat and gain 12 J of energy? 3. A gas absorbs 51 J of heat as it does 96 J of work. with explanation and solution please.arrow_forwardWhat is the specific heat of a metal with a mass of 14g,heat of 3.45kj and a change in temperature of 3.2 °carrow_forward3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY