
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
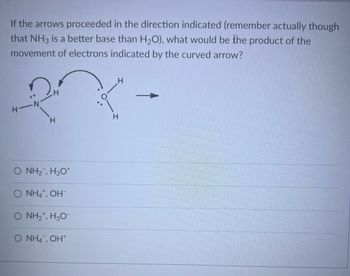
Transcribed Image Text:If the arrows proceeded in the direction indicated (remember actually though
that NH3 is a better base than H₂O), what would be the product of the
movement of electrons indicated by the curved arrow?
H-N
H
H
O NH2, H3O*
O NH4+, OH-
O NH2*, H3O-
O NH4, OH+
H
H
Expert Solution
arrow_forward
Step 1: introduce to the Bronsted Lowry theory
According to the Bronsted Lowry theory,
an acid is a compound that when dissolved in water releases hydrogen ions.
An acid is a donor of hydrogen ions.
A base is a compound that when dissolved accepts hydrogen ions, so
a base is acceptor of hydrogen ions.
The compound that is formed after accepting hydrogen ion is known as conjugate acid.
The compound that is formed donation of hydrogen ion is known as conjugate base.
Here we are required to find the products that are formed for the given reaction.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the pH of water solutions with the following characteristics. Classify each solution as acidic, basic, or neutral. (a) [H3O+] = 7.6 × 10-4 Cacidic Oneutral basic (b) [OH-] = 5.9 × 10-² acidic Oneutral Obasic (c) [H3O+] = [OH¯] acidic Oneutral Obasic (d) [H3O+] = 8.6 × 10-⁹ Oacidic Oneutral Obasic Previous - Nextarrow_forward2. a) For each compound show its conjugate base. Lone pairs have been left out but are assumed to be t Show all valence electrons. Show any resonance structures if applicable. b) Rank the conjugate bases in the order you would predict, from most stable to least stable. c) Rank the original compounds in order, from strongest acid to weakest acid. OH Br OH OH OHarrow_forwardFor the following acid-base reaction, predict which side of the equilibrium is favored. O Right-hand side O Left-hand side O Both are alkane protons, so neither side is favored.arrow_forward
- Label all of the acidic hydrogen. (Do not count any of the ones that are not shown) bns 2001320 Circle the one that is the most acidic kne noltamoino siano ws Explain, using CARDIO, why it is the most acidic. H H H H N. H. H H stasib was baie vincul CI 6057 enim09 ogmo) ons nod:6) istim relev H S WH bus il basi WH Uns 2506 A toarrow_forward2. Draw the products using curved arrows for the following acid base reaction. Label its conjugated acid-base pair. I—Z Base + N H. Acid H H searrow_forwardRank the acids in the table below from strongest (1) to weakest (4). The most acidic H atom in each acd has been highlighted. H. H. H (Choose one) (Choose one) (Choose one) (Choose one)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY