
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
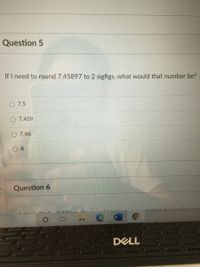
Transcribed Image Text:Question 5
If I need to round 7.45897 to 2 sigfigs, what would that number be?
7.5
7.459
7.46
8.
Question 6
口i A
W.
DELL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Choose the BEST statement from the options below. 2 3 4 5 6 7 ΤΑ 2A h 4341012 #A ies 3B 4B 5B 6B 7B 8B 98 10B 11B 128958 S 10 11 12 242 243 R FESSE 12 - &GESET 45.468 476290641.224 42906455007129 6.42 1077 112:41 1142 671 1217612760 130 131.29 22 2 M BE Hi MI 14 N !! Ta 175 1834 1852123221969022392043 204 3 Pr (145) (225 22:04 231.84 20 (237) Se 3A 4A SA 6A 7A 15 BEE -- Th H Re Ca N CITY ANAPE 13 " 115 16 2 43 3.2 PRA AR C 19% 8A =|- Ti A. Magnesium (Mg) and chlorine (Cl) are in the same GROUP and, so, will have very DIFFERENT physical and chemical properties. B. Magnesium (Mg) and chlorine (CI) are in the same PERIOD, and, so, will have very DIFFERENT physical and chemical properties. OC. Magnesium (Mg) and chlorine (CI) are in the same GROUP and, so, share SIMILAR physical and chemical properties. OD. Magnesium (Mg) and chlorine (CI) are in the same PERIOD and, so, share SIMILAR physical and chemical properties.arrow_forwardFinish the tablearrow_forwardHelparrow_forward
- Thank you, can you expand the process of how did you get the number 0.22152?arrow_forwardQUESTION 3 What are the merits and limitations of precipitation methods? Give an example where the precipitation method cannot be applied to separate two radioisotopes. For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). BIUS Paragraph Arial 10pt ... Is ABC 田田 图田用田 区arrow_forwardQuestion 5 1 pts Determine the average atomic mass for lodine with the following information (record the answer with 4 sig figs): mass % abundance 126.0 17.00% 127.0 80.00% 128.0 3.000% No new data to save. Last checked at 7:41pm Submit Quiz earch 45°F Caps Lock Num Lock F4 F5 F6 F7 F8 F9 F10 F11 % & 7 *- 5 7 8 9arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY