
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
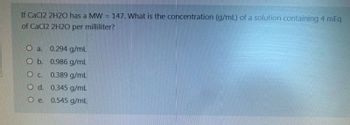
Transcribed Image Text:If CaCl2 2H2O has a MW = 147. What is the concentration (g/mL) of a solution containing 4 mEq
of CaCl2 2H2O per milliliter?
O a. 0.294 g/mL
O b. 0.986 g/mL
O c. 0.389 g/mL
O d. 0.345 g/mL
O e. 0.545 g/mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If a solute (with molar mass 55 g/mole) in a solution has a concentration of 159% mass/volume, what is its molarity? (Hint: find grams per liters first; then convert them to moles) O a. 15 mol/L O b. 0.27 mol/L OC. 2.7 mol/L O d. 1.5 mol/L O e. 0.55 mol/Larrow_forwardHow many milliliters of a 3.9 M NaCl solution would be needed to prepare each solution? a. 35 mL of a 2.4 M solution: mL b. 170 mL of a 0.79 M solution: mL < Prev 15 of 16 Marrow_forwardWhat is the mass of H2SO4 (molar mass 98 g/mol) in 400. mL of 0.100 M solution? O 3.92 g O 4.90 g 2.45 g 9.80 g MacBook Air 吕口 F3 F8 F9 F10 F4 F5 F6 F7 F1 2$ % & 2 4. 7 9- * CO 6 %#3arrow_forward
- JJ is making miso soup. 1 bowl of soup has a volume of 375 mL. The soup has 1.00 g of table salt in it (NaC1). a. What is the salt concentration of the soup in g/L? b. Express this as m/V%. c. How many molecules of NaC1 are in one bowl of JJ's miso soup?arrow_forward4. Show solution. Thanks!arrow_forward6. Show solution. Thanks!arrow_forward
- When 17.4 ml of a solute is dissolved into 25.0 ml of water, the volume of a solution becomes 28.2 ml. What is the v/v concentration of the solution? 1.38.3% 2.61.7% 3.88.7% 4.30.4% 5.69.6%arrow_forwardWhat volume of a 4.00 × 10-4 M FeSCN²+ solution would be needed to prepare 50.0 mL of a 7.20 × 10-5 M solution? O A. 20.0 mL O B. 278 mL O C. 5.56 mL O D. 9.00 mL O E. 3.60 mLarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY