
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Walmart - Hiring Ce...
Bi
F2
@
P
W
Y
F3
O
4
#
3
E
D
C
11
R
F4
144
$
4
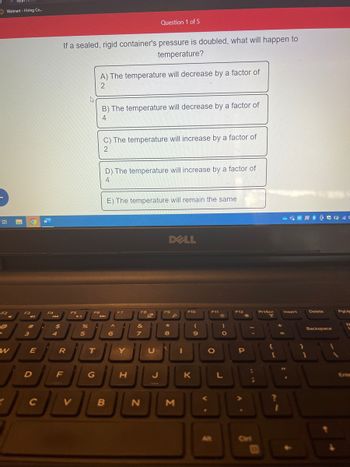
If a sealed, rigid container's pressure is doubled, what will happen to
temperature?
R
F
F5
V
%
5
A
F6
T
G
A) The temperature will decrease by a factor of
2
B) The temperature will decrease by a factor of
4
C) The temperature will increase by a factor of
2
D) The temperature will increase by a factor of
4
B
E) The temperature will remain the same
A
6
F7
A
Y
H
Question 1 of 5
F8
&
7
N
Q
UN
DELL
F9
8
F10
O
JU
K
3
9
F11
O
A
Alt
)
O
L
F12
P
:
:
;
163
PrtScr
"
{
S
[
?
Insert
1
F
F
Delete
Backspace
1
PgUp
N
Ente
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Three samples of three different gases are listed in the table below. All the samples contain exactly 1 mole of gas and are at a temperature of -10.0°C. For each sample, decide whether the gas can be reasonably described as an ideal gas. If the gas isn't ideal, decide the most important reason why it isn't. sample A B C pressure (atm) 2.8 1.1 0.86 volume (L) 10.0 20.0 30.0 ideal? O O yes no yes no yes no If not ideal, the most important reason why not: O There are attractions between the particles. The particles don't have zero volume. There are attractions between the particles. The particles don't have zero volume. O There are attractions between the particles. The particles don't have zero volume. X Sarrow_forwardA gas is collected over water at a temperature where the vapor pressure of water is known to be 22.0 mmHg. The total pressure recorded in the container is 790 mmHg. What is the pressure of the gas being collected in mmHg?arrow_forwardA sample of hydrogen gas is collected over water at 25 Celsius and the vapor pressure of the water at 25 Celsius is 24 mmHg if the barometer reading is 754 mmHg what is the partial pressure of the hydrogen gas?arrow_forward
- The following set up is arranged in a laboratory. £ Vessel 1 Vessel 2 Assuming the volume of the connecting tubing is negligible and there is no temperature change, determine the final total pressure if the two flasks were allowed to mix. If vessel 1 was initially at 4.0 atm and 1332 L, and vessel 2 was initially at 2.7 atm and 400 L.arrow_forwardThree samples of three different gases are listed in the table below. All the samples contain exactly 1 mole of gas and are at a temperature of -10.0°C. For each sample, decide whether the gas can be reasonably described as an ideal gas. If the gas isn't ideal, decide the most important reason why it isn't. sample A B C pressure (atm) 1.9 1.7 0.86 volume (L) 10.0 15.0 20.0 ideal? O yes no O yes O O no yes no If not ideal, the most important reason why not: O There are attractions between the particles. O The particles don't have zero volume. O There are attractions between the particles. O The particles don't have zero volume. There are attractions between the particles. O The particles don't have zero volume. X 5arrow_forwardThe pressure in an aerosol can is 10.2 ATM at 21.1°C the temperature is increased to 87.6°C what is the pressure of the gas at the new temperaturearrow_forward
- Three samples of three different gases are listed in the table below. All the samples contain exactly 1 mole of gas and are at a temperature of -10.0°C. For each sample, decide whether the gas can be reasonably described as an ideal gas. If the gas isn't ideal, decide the most important reason why it isn't. sample A B C pressure (atm) 1.3 0.72 0.54 volume (L) 20.0 30.0 40.0 ideal? O yes O no O yes O no O O O yes O no If not ideal, the most important reason why not: O There are attractions between the particles. O The particles don't have zero volume. O There are attractions between the particles. O The particles don't have zero volume. O There are attractions between the particles. O The particles don't have zero volume. X Śarrow_forwardThree samples of three different gases are listed in the table below. All the samples contain exactly 1 mole of gas and are at a temperature of -5.0°C. For each sample, decide whether the gas can be reasonably described as an ideal gas. If the gas isn't ideal, decide the most important reason why it isn't. sample A B C pressure (atm) 1.1 0.59 0.50 volume (L) 20.0 30.0 40.0 ideal? yes O no O yes O no O yes O no If not ideal, the most important reason why not: O There are attractions between the particles. O The particles don't have zero volume. There are attractions between the particles. O The particles don't have zero volume. O There are attractions between the particles. O The particles don't have zero volume. X 5arrow_forwardUse the information and diagram to answer the following question. A researcher carries out an investigation of a gas in a closed, inflexible container at three different temperatures. A diagram of the researcher's set up is shown. Low P Medium P High P Gas Gas Hot plate on medium 1 Hot plate off L Gas Hot plate on higharrow_forward
- The height of Mt. Everest is 29,000' and the atmospheric pressure is 253 mmHg (0.333 atm). The gas constant R is 8.314J/K*mol and the heat of vaporization of water (AHvap) is 40.8 kJ/mol. The pressure at sea level is 760 mmHg. Note 1: The P₁ is the pressure at sea level and P₂ is the pressure on Mt. Everest. Note 2: The T₁ temperature is the boiling point of water at sea level which is 100 °C or 373 K. Note 3: The term AHvap is express in kJ/mol. This must be converted to J/mol. a) Using the two component Clausius-Clapeyron equation, determine the boiling point of water on the top of Mt. Everest. InP1/P₂= AHvap/R(1/T2-1/T1) 4arrow_forwarda container is filled to a volume of 66.9 L at 29 degrees celsius. While keeping the temperature constant the volume is reduced to 19.8 L and the pressure at the end was reduced to be 7.58 atm. What was the initial pressure inside the container, in units of atm?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY