
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
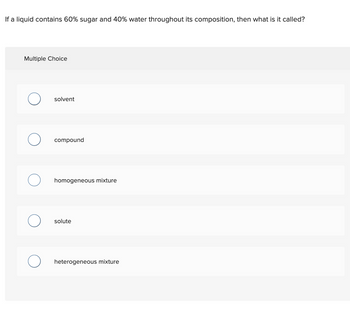
Transcribed Image Text:If a liquid contains 60% sugar and 40% water throughout its composition, then what is it called?
Multiple Choice
solvent
compound
homogeneous mixture
solute
heterogeneous mixture
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- According to the kinetic-molecular theory, gas molecules have little distance between molecules. weak interactions between molecules. less energy than molecules of a solid. strong interactions between molecules.arrow_forwardWhich state has more Kinetic energy?a) Liquid Water b) Solid water c) Water vaporarrow_forwardPotassium chloride (salt) is added to water and the mixture is stirred until no more salt dissolves. The salt that does not dissolve is allowed to settle out. Refer to the container on the left of the figure. At this point, is the solution saturated, unsaturated, or supersaturated? The water is allowed to evaporate until the volume of the solution is half the original volume. The temperature remains constant during this entire time. Nothing special is done to the solution to trick it. Refer to the container on the right of the figure. At this point, is the solution saturated, unsaturated, or supersaturated? After evaporation, is the mass of potassium chloride dissolved in solution more than, less than, or the same as the mass of potassium chloride dissolved in solution before evaporation? (YES, You are seeing more solid on the bottom of the container on the right.) After evaporation, is the concentration (molarity) of the solution more than, less than, or…arrow_forward
- How do I write out the molecular formula for Acetyl Salicyclic Acid and Salicylic Acid (SA) using the attached screenshot?arrow_forwardIF YOU WANT A PURE SUBSTANCE SEPARATED FROM IMPURITIES, WHICH SETUP WOULD YOU USE Heat IMPURE LIQUID (BOILING) O None of these OUT -COLD WATER DISTILLED LIQUID Water Out Water In -Reactants Water waarrow_forwardGasoline is composed of a variety of different liquid hydrocarbons, which do not separate as time passes. Gasoline is an example of a: A) heterogeneous mixture B) Chemical compound C) Chemical element D) Solutionarrow_forward
- True or False When a physical change occurs, a change in state occurs which indicates that a new substance has been formed.arrow_forwardThe results for the demonstration for polarity of various substances presented in the image below. Note that each vial has two liquid layers, water and oil. (Oil is a hydrocarbon chain.) Use this image to complete the following question. oil oil oil oil oil water water water water water With iodine (dark purple solid) Blank With copper sulfate (ight blue solid) With sulfur (yellow solid) With sodium chloride (no solute) (colortess, ionic solid) Classify each of the compounds on display as a polar liquid, non-polar liquid, polar solid or non-polar solid.arrow_forwardDescribe three changes in physical properties you might observe when two liquids solutions are mixed and a chemical change takes placearrow_forward
- What is the molarity of a solution containing 36.3 g of NaOH in 1.40 L? The molar mass of NaOH is 40.0 g/mol. Suppose you add 600 mL of water to the NaOH solution in the question above. What is the new molarity of the dilute NaOH solution?arrow_forwardIs the boiling point of a chemical a physical property or chemical property?arrow_forwardWhat is the average?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY