
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
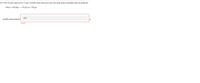
Transcribed Image Text:If 7.50 L of water vapor at 50.2 °C and 12.26 kPa reacts with excess iron, how many grams of iron(III) oxide are produced?
2 Fe(s) + 3 H,O(g)
Fe,O,(s) + 3 H,(g)
2.02
iron(III) oxide produced:
g
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5.72 A sample of zinc metal reacts completely with an excess of hydrochloric acid: Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g) The hydrogen gas produced is collected over water at 25.0°C using an arrangement similar to that shown in Figure 5.15. The volume of the gas is 7.80 L, and the pressure is 0.980 atm. Calculate the amount of zinc metal in grams consumed in the reaction. (Vapor pressure of water at 25°C = 23.8 mmHg.)arrow_forwardAmmonia burns in oxygen in the presence of a platinum catalyst to produce nitrogen monoxide and water by the following reaction: 4NH3(g) +50₂ (g) 4NO (g) + 6H₂O (1) If 9.00 g of ammonia is burned in excess oxygen, what is the mass of water that will be produced and the volume of NO gas if the reaction was carried out at 25 °C under a pressure of 100 kPa? K Periodic Table of the Elements -=||||2||×||2||81|41| Be Ca Sc Ba Fr Ra 67-71 09-103 22 Ti IN 23 24 25 41 25 27 28 29 30 Cr Mn Fe Co Ni Cu Mal 40 41 445 47 Zr Nb Mo Tc Ru Rh Pd Ag Hf Ta Series La La "Ac Th Pa Symbol Albe Viety Re Os Pt Au Hg Malay Alkaline Transition Basic Esth 13 13. Now Al Si 14 Ga Ge Noble Bas Tl Pb Bi Po At Rn 104 105 105 107 108 109 110 111 112 Rf Db Sg Bh Hs Mt Ds Rg Cn Uut "FI Uup "Lv Uus Uuo Sn Sb La The mass of water is 8.2 g and the volume of gas is 7.6 L. The mass of water is 6.4 g and the volume of gas is 11.1 L. LIBUR Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Es Fm Md No 100 102. Np Pu Am Cm Bk The mass…arrow_forwardRefer to the following equation: Mg (s) + 2HNO3(aq) -> Mg(NO3)2 (aq) + H2 (g) A reaction between 0.359 grams of Mg and 21.12mL of a 0.450M HNO3 solution was conducted in a room witht he following conditions: 27.5 degrees Celius, 723mmHg of atospheric pressure. If .15mL of H2 were collected over water (by the water displacement method), calculate the percent yeild of the reaction. (Pressure H2O at 27.5 degrees Celius = 27.82 mmHg)arrow_forward
- Sulfur dioxide is an intermediate in the preparation of sulfuric acid. What volume (L) of SO 2 at 343*C and 1.21 atm is produced by burning 1.00 Kg of sulfur in oxygen? S 4(s) + 4O 2(g)--> 4SO 2(g)arrow_forwardHow much pressure in kPa does 4.00 moles of nitrogen gas at 30.0°C exert on a container that holds 55.0 L of gas?arrow_forwardWhat mass of magnesium is required to react with 265.0 mL of 0.75M HCl at 26 degrees Celsius and 150.00 kPa to produce 1.15 L of hydrogen gas? (Water vapour pressure at this temperatue is 3.36 kPa) Select final answer from the following: - 19.4 g - 1.69 g - 1.65 g - 19.0 garrow_forward
- The air bags in cars inflate through the explosive decomposition of a solid to produce a gas. The simplified version of the reaction that takes place in most air bags is shown. 2NaN3(s) -> 2Na(s) + 3N2(g) (a) If a typical air bag has a volume of 67.0 L when inflated, how much NaN3 (in grams) is required to inflate an air bag at STP? (b) Would you want to rely on an air bag that contained only 40.0 g NaN3? Explain.arrow_forwardI have a hard timearrow_forwardUse the reaction below to calculate the mass of iron that must be used to obtain 15.1 L of hydrogen at STP. 3Fe(s) + 4H2O → Fe3O4(s) + 4H2(g)arrow_forward
- The combustion of gasoline (octane, C8H18) as shown in the following equation is what powers most cars. CO2 is a major cause of climate change. What mass of CO2 is produced by one tank of gasoline that holds 1.63x104 L at a temperature of 80.0°C and a pressure of 101.3 kPa? Plants use the CO2 produced to make glucose and O2. A mature tree can absorb 22 kg of CO2 a year. How many trees are required to absorb the CO2 produced by one tank of gas? 2 C8H18 (g) -> 25 O2 (g) -> 16 CO2 (g) -> 18 H2O (g)arrow_forwardGaseous iodine pentafluoride, IF, can be prepared by the reaction of solid iodine and gaseous fluorine: 1,(s) + 5F,(g) → 2IF,(g) A 10.00 L flask is charged with 14.0 grams of I, and 2.00 atm of F, 25°C. The flask is heated to 100°C until one of the reagents is completely consumed. What will be the total pressure (in atm) of the final products in the flask at 100°C? (Assume ideal gas behavior). atarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY