
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
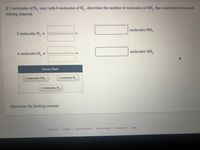
Transcribed Image Text:If 3 molecules of N, react with 6 molecules of H., , determine the number of molecules of NH, that could form from each
starting material.
molecules NH,z
3 molecules N, ×
molecules NH,z
6 molecules H, x
Answer Bank
2 molecules NH,
1 molecule N,
3 molecules H,2
Determine the limiting reactant.
| contact us
|help
about us
| privacy policy terms of use
careers
MacBo
||

Transcribed Image Text:Determine the limiting reactant.
ON,
OH,
-2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following reaction, 4.80 grams of hydrogen gas are allowed to react with 9.37 grams of ethylene (C,H4). hydrogen (g) + ethylene (C¿H4) (g)- - ethane (C,Hg) (g) What is the maximum amount of ethane (C,Hô) that can be formed? | grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? | gramsarrow_forwardHydrazine (N₂H4), a component of some rocket fuels, is industrially produced using the following reaction. 2 NH3 + H₂O2 → N₂H4 + 2 H₂O Molar masses (in g mol-¹) NH3 17.03 Number % H₂O₂ 34.02 N₂H4 32.05 A: What is the percent atom economy for the synthesis of hydrazine? Enter a percentage accurate to 3 significant figures. Do not enter the "%" symbol. The E-factor is greater than 1. The E-factor is less than 1. H₂O 18.02 B: What is the E-factor when a reaction between 170.3 g of NH3 and 170.1 g of H₂O₂ produces 160.3 g of N₂H4 and 90.1 g of water? The E-factor is 0.arrow_forward4arrow_forward
- For each of the following unbalanced equations, calculate how many grams of each product would be produced by complete reaction of g of the reactant indicated in boldface. Indicate clearly the mole ratio used for the conversion. TiBr₄(g)+H₂(g)→Ti(s)+HBr(g) SiH₄(g)+NH₃(g)→Si₃N₄(s)+H₂(g) NO(g)+H₂(g)→N₂(g)+2H₂O(℩) Cu₂S(s)→Cu(s)+S(g)arrow_forwardA student runs the reaction represented by the equation below. What mass of H2 gas would be produced if the student begins with 5.63 grams of each reactant? Round to the nearest 0.01 and remember to include both units and substance in your answer!!! Zn + HCl --> ZnCl2 + H2arrow_forwardded for this question. When iron(III) oxide reacts with aluminum, aluminum oxide and iron are produced. The balanced equation for this reaction is: Fe2O3 (8)+2Al(s) → Al2O3 (8) + 2Fe(8) If 4 moles of aluminum react, The reaction consumes The reaction produces moles of iron(III) oxide. moles of aluminum oxide and moles of iron.arrow_forward
- 4. A mixture of glucose and oxygen reacts in acroblc respiration. The right-hand box below shows a particle diagram of the moles of substances present after the reaction is complete. On a piece of paper draw the "Before" box as shown and draw a particle diagram of the reactant molecules that produced the mixture shown on the right. Key = 02 = H20 = CO2 = CeH1206 %3D Before After You will need to draw a diagram to answer this question. On a piece of paper, draw the "Before" box as shown, and then draw a particle diagram of the reactant molecules that produced the mixture shown on the right.arrow_forwardReactants Products Before reaction 3 mol N, 6 mol H, O mol NH, After reaction 1 mol N2 O mol H2 4 mol NH, Reaction in presence of limiting reagent. Ammonia, NH3, is created from the reactants N2 (shown in green) and H2 (shown in white) according to the following balanced chemical equation. In the example above you begin with 3 mol of nitrogen gas and 6 mol of hydrogen gas. N2+3H2----> 2NH3 Which is the limiting reactant? You can tell that hydrogen is the limiting reactant because all of it was used up, but there is some leftover (excess) nitrogen in the diagram when the reaction is complete Since the mole ratios match up perfectly between the reactants neither of them is limiting. You can tell that ammonium is the limiting reactant because there were 0 moles of it before the reaction started. You can tell that nitrogen gas is the limiting reactant because there were fewer moles of it with which to begin.arrow_forward[References] TUTOR Chemical Equations: Gram/Gram Calculation Consider the reaction: SOe) + H;O(1) → H3SO;( Given an initial mass of 15.3 g SO, an excess of H50, and assuming that all of the reactant is converted to product(s), and none is lost, calculate the mass (g) of H7SO3 produced by the reaction.arrow_forward
- Mercury can be isolated from its ore by the reaction: 4 HgS + 4 CaO → 4 Hg + 3 CaS + CaSO4 How many moles of CaS are produced from 2.5 mol CaO? Enter your answer in moles into the first answer field in accordance with the question statement. mol CaS How many kilograms of Hg are produced along with 9.75 mol of CaSO4? Enter your answer in kilograms into the second answer field in accordance with the question statement. kg Hgarrow_forwardFor the reaction shown, find the limiting reactant for each of the initial quantities of reactants. express all answers as chemical forumals 2Li(s)+Br2(l)→2LiBr(s) -1 molLi ; 1 molBr2 -1.8 molLi ; 1 molBr2 -2.2 molLi ; 1 molBr2 -arrow_forwardHow do i find the theoretical yield and how much water is produced?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY