
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
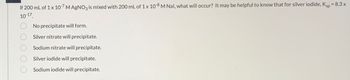
Transcribed Image Text:If 200 mL of 1 x 10-7 M AgNO3 is mixed with 200 mL of 1 x 10-8 M Nal, what will occur? It may be helpful to know that for silver iodide, Ksp = 8.3 x
10-17.
No precipitate will form.
Silver nitrate will precipitate.
Sodium nitrate will precipitate.
Silver iodide will precipitate.
Sodium iodide will precipitate.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 19 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Classify each reaction according to whether a precipitate forms. Pricipitate forms Precipitate does not form Mg(NO,),+NaCI NANO3 + NACIO4 Cu(NO3)2+N2CI Ca(NO3)2 + Na,CO3 AgNO3+NAOH Fe(NO3)2 + NazPO4 Pb(NO3)2+Nal Answer Bankarrow_forwardThe solubility of lead(II) iodide is 1.39 x 10-3 mol/L at a certain temperature. What is the solubility product, Ksp, for lead(II) iodide.arrow_forward13. The Kp of SrCO3 is 1.6 x 10. What is the solubility of SrCO3 (in mol/L) in pure water? sp 14. The KOH(aq) concentration is 1.2 x 10³ M. What concentration of Fe³ must be added to just start forming a precipitate Fe(OH),(s)? 3 15. The solubility of LaF¸ in water is 1.8 x 10°³ g/L. What is the value of K? sp 16. The K of PbBг, is 4.6 x 106. What is the solubility of PbBr₂ (in g/L) in pure water? sparrow_forward
- The Solubility Product Constant for barium phosphate is 1.3 × 10-29⁹. Solid potassium phosphate is slowly added to 50.0 mL of a 0.0350 M barium acetate solution. The concentration of phosphate ion required to just initiate precipitation isarrow_forward15. Calculate the solubility product constant for aluminum hydroxide (78 g/mol). Its molar solubility is 2.9×10−9mole per liter at 25°C a 9.8 ×10−26 b 4.9 ×10−26 c 7.1 ×10−35 d 2.1 ×10−34 e 1.9 ×10−33arrow_forwardKSP for Copper Sulfide is: 7.9 × 10-37 KSP for Copper Hydroxide is: 1.6 × 10-19arrow_forward
- When 25.0 mL of a 9.81×10-4 M sodium hydroxide solution is combined with 22.0 mL of a 3.54×10-4 M nickel(II) sulfate solution does a precipitate form? fill in the blank 1 (yes or no)For these conditions the Reaction Quotient, Q, is equal to ______ .arrow_forwardA solution contains 1.22x10-2 M lead nitrate and 1.05x10-² M silver acetate. Solid sodium chloride is added slowly to this mixture. What is the concentration of silver ion when lead ion begins to precipitate? [Ag*]= Submit Answer Retry Entire Group 7 more group attempts remainingarrow_forwardA solution contains 1.45×10-2 M calcium nitrate and 1.29×10-2 M silver acetate.Solid potassium sulfate is added slowly to this mixture.What is the concentration of calcium ion when silver ion begins to precipitate?[Ca2+] = Marrow_forward
- A solution contains 1.45x102 M sodium sulfate and 6.19×10-3 M sodium phosphate. Solid lead nitrate is added slowly to this mixture. What is the concentration of phosphate ion when sulfate ion begins to precipitate? [phosphate] =| Marrow_forwardyou would like to remove cadmium ions from a waste stream using precipitation. The final concentration of cadmium needs to be less than 0.001 mg/L. identify a chemical that could be used to precipitate cadmium out of solution to meet this goal. Show calculations and prove if it can be calculated.arrow_forwardAssume the solubility product of Mg(OH)2 is 1.3 x 10 at a certain temperature. What minimum OH concentration must be attained (for example, by adding NaOH) to decrease the Mg2+ concentration in a solution of Mg(NO3), to less than 1.2 x 10-10 M. 3/2 x 10 M (Enter your answer in scientific notation.) Next > < Prev 18 of 20 **** .*****arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY