
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
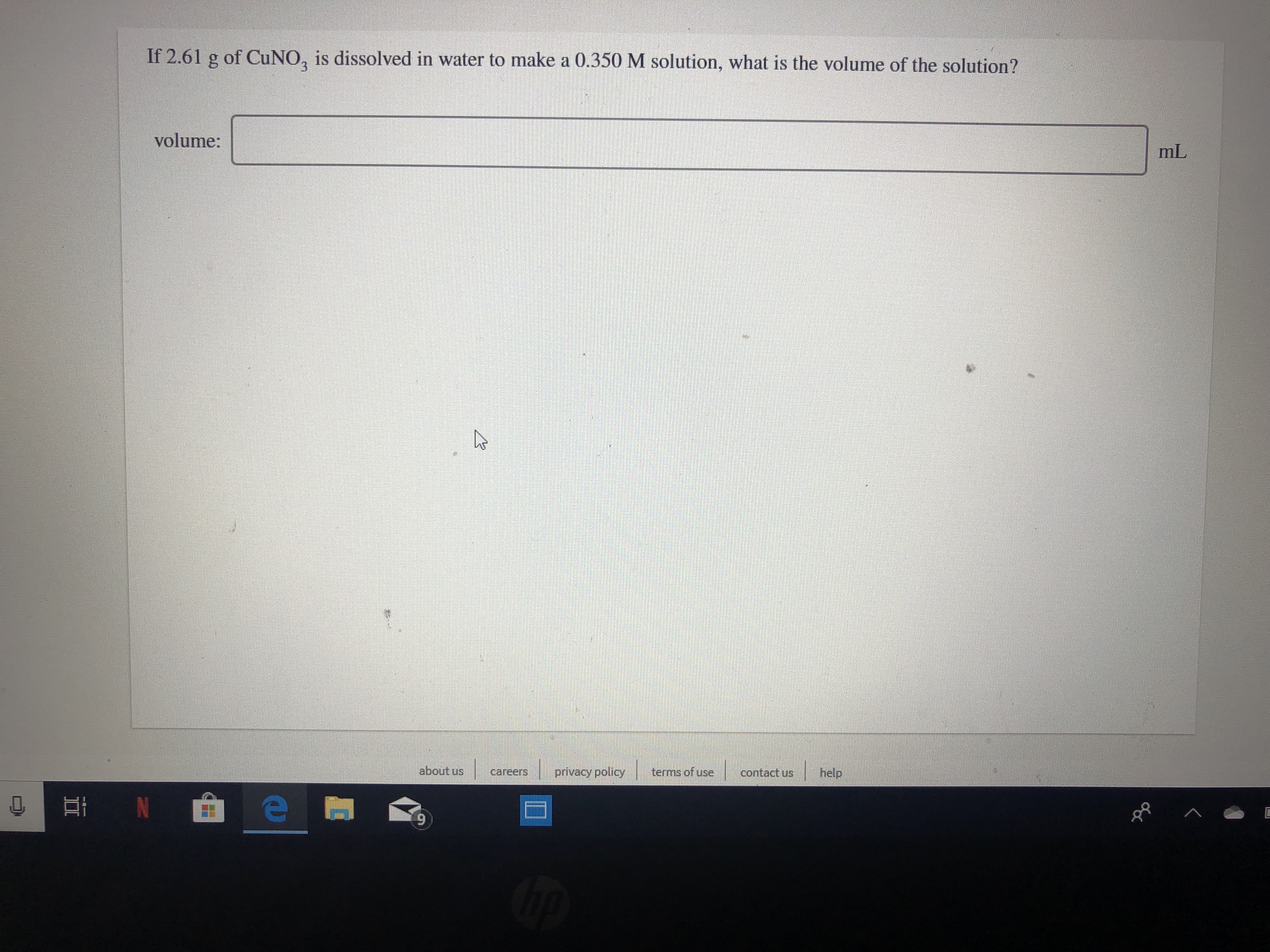
Transcribed Image Text:If 2.61 g of CuNO, is dissolved in water to make a 0.350M solution, what is the volume of the solution?
volume:
mL
about us
help
privacy policy
terms of use
careers
contact us
H N
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- What mass of HCl is contained in 45.0 mL of an aqueous HCl solution that has a density of 1.19 g cm-3 and contains 37.21% HCl by mass?arrow_forwardA solution is 0.1% by mass calcium chloride. Therefore, 100. g of the solution contains g of calcium chloride.arrow_forwardA cough syrup contains 5.0% ethyl alcohol, C2H5OH, by mass. If the density of the solution is 0.9928 g/mL, determine the molarity of the alcohol in the cough syrup.arrow_forward
- A throat spray is 1.40% by mass phenol, C6H5OH, in water. If the solution has a density of 0.9956 g/mL, calculate the molarity of the solution.arrow_forwardHow do we define the mass percent composition of a solution? Give an example of a solution, and explain the relative amounts of solute and solvent present in the solution in terms of the mass percent composition.arrow_forwardWhat volume of a 0.33MC12H22O11 solution can be diluted to prepare 25 mL of a solution with a concentration of 0.025 M?arrow_forward
- Consider this question: What mass of a concentrated solution of nitric acid (68.0% HNO3 by mass) is needed to prepare 400.0 g of a 10.0% solution of HNO3 by mass? (a) Outline the steps necessary to answer the question. (b) Answer the question.arrow_forwardFind the molarity of a 40.0% by mass aqueous solution of sulfuric acid, H2SO4, for which the density is 1.3057 g/mL.arrow_forwardOne mole of CaCl2 is represented as where represents Ca and represents Cl. Complete the picture showing only the calcium and chloride ions. The water molecules need not be shown. What is the molarity of Ca2+? of Cl-?arrow_forward
- A solution is prepared by diluting 0.7850 L of 1.262 M potassium sulfide solution with water to a final volume of 2.000 L. (a) How many grams of potassium sulfide were dissolved to give the original solution? (b) What are the molarities of the potassium sulfide, potassium ions, and sulfide ions in the diluted solution?arrow_forwardIf you dilute 20.0 mL of a 3.5M solution to make100.0 mL of solution, what is the molarity of the dilutesolution?arrow_forwardWhen a solution is diluted by adding additional solvent, the concentration of solute changes hut the amount of solute present does not change. Explain. Suppose 250. mL of water is added to 125 mL of 0.55 1 M NaCl solution. Explain how you would calculate the concentration of the solution after dilution.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER