
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
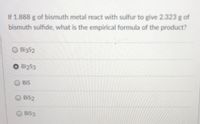
Transcribed Image Text:If 1.888 g of bismuth metal react with sulfur to give 2.323 g of
bismuth sulfide, what is the empirical formula of the product?
O Bi3S2
Bi2S3
BIS
BIS2
BIS3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Sulfuric acid can be prepared starting with the sulfide ore, cuprite (Cu₂S). If each S atom in Cu₂S leads to one molecule of H₂SO4, what is the theoretical yield of H₂SO4 from 3.20 kg of Cu₂ S? Mass= garrow_forwardDisulfur dichloride, which has a revolting smell, can be prepared by directly combining Se and Cl₂, but it can also be made by this reaction: 3 SC1₂ (1) + 4 NaF (s) → SF4 (g) + S₂ Cl₂ (l) + 4 NaCl(s) Calculate the mass of SC12 needed to react with excess NaF to prepare 1.37 g S₂ Cl2 if the expected yield is 46%. Mass of SC12 = garrow_forwardHow many moles of H₂O can be formed from 5.28 × 10²³ atoms of NH₃ from the following equation? 4 NH₃(g) + 5 O₂(g) → 4 NO(g) + 6 H₂O(g)arrow_forward
- Small quantities of oxygen can be prepared in the laboratory by heating potassium chlorate, KClO3(s).KClO3(s). The equation for the reaction is 2KClO3⟶2KCl+3O22KClO3⟶2KCl+3O2 Calculate how many grams of O2(g)O2(g) can be produced from heating 67.3 g KClO3(s).arrow_forwardConsider the reaction. Pb(SO4)2 + 2 Zn → 2 ZnSO4 + Pb If 0.422 mol of zinc reacts with excess lead(IV) sulfate, how many grams of zinc sulfate will be produced in the reaction?arrow_forwardHow many moles of H₂O can be formed from 7.44 × 10²³ molecules of NH₃ from the following equation? 4 NH₃(g) + 5 O₂(g) → 4 NO(g) + 6 H₂O(g)arrow_forward
- For the chemical reaction CaI2+2AgNO3⟶2AgI+Ca(NO3)2 what mass of silver iodide is produced from 1.99 mol of calcium iodide?arrow_forwardFor the chemical reaction 2KI+Pb(NO3)2⟶PbI2+2KNO3 what mass of lead(II) iodide is produced from 2.61 mol of potassium iodide?arrow_forwardHow many moles of H₂O can be formed from 3.02 × 10²³ molecules of NH₃ from the following equation? 4 NH₃(g) + 5 O₂(g) → 4 NO(g) + 6 H₂O(g)arrow_forward
- After running various experiments, you determine that the mechanism for the following reaction is bimolecular. Br + OH - HO. Using this information, draw the correct mechanism in the space below. Br + Br Add/Remove step 5 � Click and drag to start drawing a structure.arrow_forwardFor the chemical reaction 2KI+Pb(NO3)2⟶PbI2+2KNO32KI+Pb(NO3)2⟶PbI2+2KNO3 what mass of lead(II) iodide is produced from 2.072.07 mol of potassium iodide?arrow_forwardSmall quantities of oxygen can be prepared in the laboratory by heating potassium chlorate, KCIO3(s) The equation for the reaction is 2KCIO3 2KCI + 3 O2 Calculate how many grams of O2(g) can be produced from heating 76.1 g KCIO3(s) g O2 mass:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY