
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Teas fille
44'F
PDF
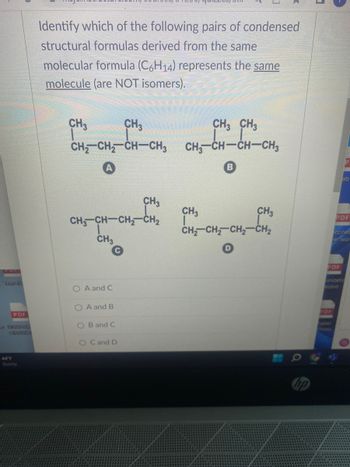
Identify which of the following pairs of condensed
structural formulas derived from the same
molecular formula (C6H14) represents the same
molecule (are NOT isomers).
CH3
|
CH3
CH,CH, CH—CH3
A
CH3CH–CH2CH,
CH3
O A and C
O A and B
CH3
OB and C
O C and D
CH3 CH3
CHICH—CHCH3
B
CH3
CH3
CH₂ CH₂ CH₂ CH₂
3
PDF
PDF
Saster
ab
ccines
er wor
ecord
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following representation of 1,2-dimethylcyclohexane.Which of the following describes the molecule represented? What is the molecular formula (in the order CH) of the compound?arrow_forwardDraw the line-angle (skeletal) notation of the saturated (maximum possible number of hydrogen atoms attached to the carbon atoms) hydrocarbon chain that is included in the R group. о CH₂O-C-R о CH₂OH 0 R-C-ONa+ о = CH-O-C-R 3 NaOH CH-OH R-C-O Na о 0 = CH₂O-C-R CH₂OH R-C-ONa+arrow_forwardRank the conformations of n-butane with reference to its potential energy from the most stable to the least stable. Rank from the most stable to the least stable.To rank items as equivalent, overlap them.arrow_forward
- What is the correct name of the molecule with the formnulas CH; CH, CH, CH, CH, CH2 CH3? Heptane Octane Butane Hexane Pentanearrow_forwardConsider the following representation of 1,2-dimethylcyclopentane.Which of the following describes the molecule represented? cis or trans isomer or noneWhat is the molecular formula (in the order CH) of the compound?arrow_forward6.arrow_forward
- Give the name for the structure in the image shown: CH,CH, CH,CH, CH3CCH,CH CH3 CH3 O 2,4-dimethyl-2,4-diethylbutane O 3,5,5-trimethylheptane O 1,3-dimethyl-1,3-diethylbutane O 3,3,5-trimethylheptanearrow_forwardhi can you name/draw the moleculearrow_forwardGive the name for each of the following compounds: Structural Formula Name Cнз .CH2 CH сH-ҫ—сHҙ CHz CH2 CHз CHз Cна -CH CH2- -CH снз CH2 CH2 CH-CH2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY