
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
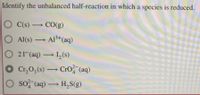
Transcribed Image Text:Identify the unbalanced half-reaction in which a species is reduced.
O C(s) CO(g)
O Al(s) Al3+(aq)
-
O 21(aq) I,(s)
O Cr,0,(s) CrO?"(aq)
O so (aq) H,S(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Consider the following half-reactions: F2(g) + 2 e ® 2 F"(aq) E° = +2.87 V Cu2*(aq) + 2 e¯ ® Cu(s) Sn2*(aq) + 2 e¯ ® Sn(s) Al3*(aq) + 3 e ® Al(s) Na*(aq) + e ® Na(s) E° = +0.337 V E° = -0.14 V E° = -1.66 V E° = -2.714 V Which of the above compounds or ions are able to reduce Al 3*? Oa. F', Cu, and Sn Ob. F2 and F OC. F2, Cu2+ Od. Na only and Sn²+ e. Na* and Naarrow_forward3) Calculate AG° Co* pe + 2es → Coc {Detailed calculation with units is a MUST} (aq)arrow_forwardExplain step by step and solvearrow_forward
- Balance the following half-reactions by adding the appropriate number of electrons (e-). Then, classify each reaction as an oxidation or reduction half-reaction. Note that for each of the four reactions, one of the gray boxes will be left blank and the other will be filled with electron(s). Use the symbol e- to represent an electron. Part 4 + 12(s) + 6H2O(1) > 2I0-3 + 12H+(aq) + --arrow_forwardA certain metal è forms a soluble sulfate salt ₂(so). Suppose the left half cell of a galvanic cell apparatus is filled with a 5.00 M solution of M₂(SO4)3 and the right half cell with a 250. mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 35.0 °C. Which electrode will be positive? O left Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. O right What voltage will the voltmeter show? Assume its positive lead is connected to the positive electrode. 0 x10 Xarrow_forwardq19arrow_forward
- Balance the below unbalanced redox reaction in acidic media. In the balanced redox reaction, there are ( Select ] water molecules in the reactants and [: [ Select ] AIO2 ions in the products. NO2 (aq) + Al (s) → NH4* (aq) + AIO2 (aq)arrow_forward(19) Consider the following redox reaction that occurs in a voltaic cell: Cr2+ (aq) + Cu²+ (aq) → Cr³+ (aq) + Cut (aq) If the cell is operating at 25°C, what is the value of the equilibrium constant (Keq)? (A) 1.41 x 10¹1 1.19 x 1018 5.53 x 105 2.79 x 1033 1.81 x 10-6 (B) (C) (D) (E)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY