
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
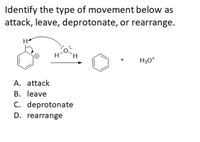
Transcribed Image Text:Identify the type of movement below as
attack, leave, deprotonate, or rearrange.
H30*
A. attack
B. leave
C. deprotonate
D. rearrange
Expert Solution
arrow_forward
Step 1
Solution
Benzene is very vulnerable to electrophilic substitution reactions compared to chemical reactions because it loses its aromaticity throughout addition reaction. As benzine contains delocalized electrons spanning over carbon atoms within the ring, it's extremely engaging to electrophiles and is additionally extremely stable to electrophilic substitutions. Generally, the electrophilic substitution reaction of benzine may be a three-step method involving:
Generation of the electrophile.
Intermediate carbocation formation.
Removal of a nucleon from carbocation intermediate.
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following is NOT true of the transition state? O a. It is unstable and dissociate into products O b. Old bonds are weakened and new bonds begin to form O c. It has a greater energy than the products O d. It cannot dissociate into products without needing any additional energy е. It has a greater energy than the reactantsarrow_forward25. Which type of reaction is indicated by a positive AH? a reversible reaction O a spontaneous reaction an exothermic reaction an endothermic reactionarrow_forwardWhat is the product of the following reaction? -NHCH, H,0, H heat но NHCH, NHCH, A. В. CH,NH,"cr CH, CH,NH,"cr D. CH,NH, "cr O A OB O D MacBook Pro G Search or type URL $ & * 4 6. 7 8. E T. Y U H Karrow_forward
- Nonearrow_forwardYou experimentally determine the rate of reaction at different temperatures while keeping the initial concentrations of the reactants constant. What relationship would you expect to find between temperature and rate? A. As temperature increases, rate decreases. B. As temperature increases, rate increases. C. Temperature has no effect on the rate of a chemical reaction. D. The relationship depends how many reactants are present.arrow_forward16. A reaction mechanism proposes three step reactions for the overall reaction and the energy diagram shows three activated states. From the proposed mechanism and energy diagram how many reaction intermediates are expected to form?A. 3 B. none C. 1 D. 2 E. 4arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY