
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
None
Transcribed Image Text:2+
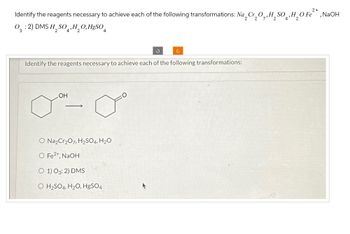
Identify the reagents necessary to achieve each of the following transformations: Na 2 Cr₂O,,H₂SO‚H₂O Fe²*, NaOH
03:2) DMS H2SO4, H2OHgSO4
Identify the reagents necessary to achieve each of the following transformations:
LOH
O Na2Cr2O7, H2SO4, H2O
O Fe2+, NaOH
O 1) O3: 2) DMS
O H2SO4, H2O, HgSO4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given the following data: 4C(s) + 4H2(g) + O2(g) → CH3CH2OCOCH3(l) ΔH°=-480.0 kJ CH3CH2OH(l) + O2(g) → CH3COOH(l) + H2O(l) ΔH°=-492.0 kJ 2C(s) + 3H2(g) + 1/2O2(g) → CH3CH2OH(l) ΔH°=-278.0 kJ H2(g) + 1/2O2(g) → H2O(l) ΔH°=-286.0 kJ calculate ΔH° for the reaction:CH3COOH(l) + CH3CH2OH(l) → CH3CH2OCOCH3(l) + H2O(l)arrow_forwardChoose the correct product !arrow_forwardquestion 21 only pleasearrow_forward
- وچی NaOH, H2O, haat NaOH, H2O, heat پہلے 0 Na Na HO OHarrow_forward[References] Use the References to access important values if needed for this question. Expressing amounts of energy in different energy units is necessary to solve many chemistry problems. For practice, complete the following table. The Joule (J) is the SI unit of energy. 1 calorie (cal) = 4.184 J J cal kJ 568 149 0.807 Submit Answer Try Another Version 10 item attempts remaining pt pt pt Previous Ne re to search DELLarrow_forwardEndothermic has a -AH True Falsearrow_forward
- !!!arrow_forwardWhat is the standard heat of formation of C2H4 H2+1/2O2>H2O. -242kj/mol 2C+3H2+1/2O2>C2H5OH. -273kj/mol C2H4+H20>C2H5OH. -88kj/mol 2C+2H2>C2H4arrow_forward2H9O (s) 2Hg (s) + 02 (g) O synthesis O decomposition O hydrocarbon combustion O single replacement O double replacementarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY