Related questions
Concept explainers
Question
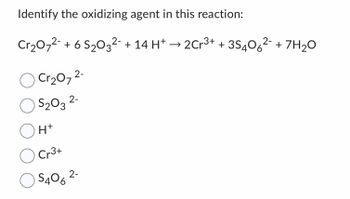
Transcribed Image Text:Identify the oxidizing agent in this reaction:
Cr₂O7²- + 6 S₂03²¯ + 14 H+ → 2Cr³+ +35406²- + 7H₂O
Cr₂O7²-
$₂03 2-
O
OH+
O Cr3+
O S406
2-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.