
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Identify the IUPAC name of the given compound.
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
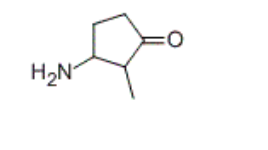
Transcribed Image Text:The image illustrates the chemical structure of proline, an amino acid. Proline is unique among the standard amino acids due to its cyclic structure, which significantly influences its role in proteins.
### Description of the Chemical Structure
1. **Ring Structure**: Proline consists of a five-membered ring structure. This ring is formed by the backbone of the amino acid, where the alpha carbon is connected to the side chain forming the ring.
2. **Amino Group (NH2)**: Attached to the alpha carbon is an amino group (NH2). In proline, this amino group is part of the ring rather than being free-standing.
3. **Carboxyl Group (C=O)**: Another critical functional group in proline is the carboxyl group (C=O). The carbon in the carboxyl group is double-bonded to an oxygen atom. This carboxyl group defines it as an amino acid.
### Importance of Proline
Proline plays a crucial role in the structure of proteins. The rigidity of the ring causes a constraint in the bond angles, which impacts the overall folding and conformation of the protein. Proline is often found in turns of protein structures, contributing to the protein's final 3D shape.
Understanding the structure and function of proline is essential for studying protein chemistry and biochemistry, as its unique properties have significant implications for protein stability and function.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Be sure to answer all parts. Give the IUPAC name for the following compound: CH3 CH3 -dimethylcyclopentanone dimethylcyclopentanal dimethylcyclopentanone cycloheptanone isocycloheptanonearrow_forwardWhat is the product after cyclopropanol undergoes dehydration?arrow_forwardWhat is the IUPAC name of the following compound? a) 2-methyl-3-amino-1-cyclopentanone b) 3-amine-2-methyl-1-cyclopentanone c) 2-methyl-3-aminocyclopentanone d) 3-amino-2-methylcyclopentanonearrow_forward
- The IUPAC name for the following molecule is: b O N-Methyl-2-methyl-1-butamine N-Butylethanamine. N-Butylethanamide N-Ethylbutanamine N-Ethylbutanamidearrow_forwardWhat time of intermolecular interactions occur when the tertiary amine of Tapentadol binds to its drug target?arrow_forwardhelparrow_forward
- What steps would be used to synthesize an organic amine? Group of answer choices Ketone + oxidizing agent →carboxylic acid + ammonia →organic amine Alkene + acid → alcohol + ammonia →organic amine Ether + acid →ester + ammonia →organic amine Alkane + acid → alcohol + ammonia →organic amine Carboxylic acid + alcohol →ester + ammonia →organic amine Aldehyde + oxidizing agent →carboxylic acid + ammonia → organic aminearrow_forward2arrow_forwardWhat is the IUPAC name of the following compound? Multiple Choice 4-methylheptan-4-amine N-propylpentan-2-amine 2-propylhexan-3-amine 1-methyl-N-propylpropan-1-aminearrow_forward
- 2. Draw the product when each of the amines acts as a base when combined with water. Name the ion that forms. a) CH-CHL- Nith + Hy0 b) CHy-NH-CH CHy + HgOarrow_forwardWrite the common name for each amine. NH name: name:arrow_forwardWhat is the IUPAC name for the following compound? CH3 CH3CH2CHCH2COOH isohexanoic acid 3-methylpentanoic acid 2-methylpentanoic acid B-methylvaleric acid 3-methylvaleric acidarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY