
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
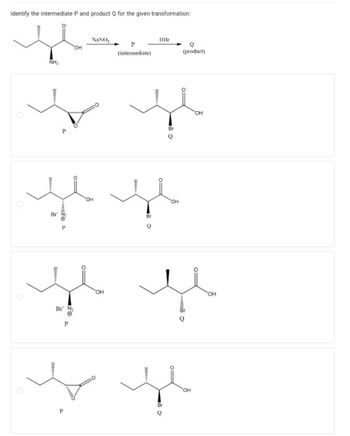
Transcribed Image Text:Identify the intermediate P and product Q for the given transformation:
H
NH₂
M
- P
Br N₂
NaNO₂
OH
A
P
(intermediate)
B
H
Br
****
HBr
Br
Q
(product)
OH
Br
O:
je je
OH
OH
Br N₂
P
جديد
OH
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the rate equation for the reaction in the box? Br CH,ONa -CH3 CH3 CH;OH H + CH,OH + Br CH;O: O Rate - k (RBr|[CH3OH) Rate - k (RBr) Rate - k [RBr][NaOCH3) O Rate - k [CH;OH]arrow_forwardChloromethane reacts with dilute sodium cyanide (Na+ -C‚N) chloromethane cyanide acetonitrile chloride. When the concentration of chloromethane is doubled, the rate is observed to double. When the concentration of cyanide ion is tripled, the rate is observed to triple. Write the rate equation for this reaction.arrow_forwardIs this correct?arrow_forward
- •OOH H H atom abstraction Consider the hydrogen atom abstraction step in an autooxidation mechanism, as shown above. Which of the following represents the intermediate that is formed in this step? OOH ? .00.arrow_forwardQUESTION 1 Answer the question below the reaction. + Excess NH4Cl In the first step of the reaction mechanism, which of the following is formed? CH3 O H3C CI H3C H3C O S OH H₂C CH3 CH3 CH3 OH CH3 -OH2 CH3 OH CH3 CH3 H₂SO4 + CI ка +H2Oarrow_forward6) Correctly complete each reaction, naming the reactants and products не он он Н+ онarrow_forward
- An elevated level of the enzyme alkaline phosphatase (ALP) in human serum is an indication of possible liver or bone disorder. The level of serum ALP is so low that it is very difficult to measure directly. However. ALP catalyzes a number of reactions, and its relative concentration can be determined by measuring the rate of one of these reactions under controlled conditions. One such reaction is the conversion of p-nitrophenyI phosphate (PNPP) to p-nitrophenoxide ion (PNP) and phosphate ion. Control of temperature during the test is very important; the rate of the reaction increases 1.47 times if the temperature changes from 30 C to 37 C. What is the activation energy for the ALP-catalyzed conversion of PNPP to PNP and phosphate?arrow_forwardproduct obtained and draw the mechanism of its formation. Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges w arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. DDC H EXP CONT ? L W OH H―CI: A ΔΟ CE CI :CI: H―CIAarrow_forwardPlease explainarrow_forward
- Consider the reaction below. If the concentration of the nucleophile is doubled, the rate of the reaction will If the concentration of the alkyl halide is doubled, the rate of the reaction will H₂O x CI OH (c) double, not change (d) triple, triple (a) not change, double(b) double, double (e) not change, not changearrow_forwardA reversible step in a mechanism indicates that the step is the rate -determining step in the mechanism. True Falsearrow_forwardConsider the given reaction in which NC−NC− is the nucleophile and CH3CNCH3CN is the solvent. The reactant molecule has a structure with solid and dashed wedge bonds. A solid wedge () is used to show the bond that is above the plane of the paper, and a dashed wedge () is used to show the bond that is behind the plane of the paper. Draw the product of the following reaction:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax