
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Identify the
Group of answer choices
ketone , ether, cyclic hydrocarbon
carboxylic acid , cyclic hydrocarbon
ester, aromatic
ketone, ether, aromatic
ester, cyclic hydrocarbon
carboxylic acid, cyclic hydrocarbon
carboxylic acid, aromatic
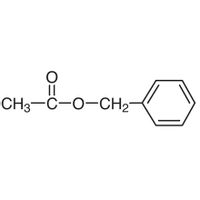
Transcribed Image Text:||
CH3-C-O-CH2-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each of the following, circle the portion of the molecule that determines its functional group classification and state whether the functional group is an acyl halide, aldehyde, amide, anhydride, carboxylic acid, ester, ketone, lactam, lactone, or nitrile. Then, unless otherwise indicated, write both the IUPAC and common name of the molecule.arrow_forwardIdentify the different functional groups in the following molecules using names from the table below. (If there are fewer than 3 different functional groups, leave an appropriate number of answer boxes empty.)arrow_forwardClassify each of the molecules according to its functional grouparrow_forward
- Write two complete, balanced equations for each of the following reactions, one using condensed formulas and one using Lewis structures. Ethyl ammonium chloride is added to a solution of sodium hydroxide.arrow_forwardQuestion 17 of 20 What is the major structural difference between PGE and PGF? A) PGE has a carboxylic acid group and PGF does not. B) PGE has no carbon=carbon double bonds, while PGF does. C) PGE has a ketone group on carbon 9, while PGF has an alcohol group at the same location. D) PGE has a 5-membered carbon ring, while PGF has a 6-membered carbon ring. E) PGE has no alcohol groups, while PGF does.arrow_forwardIdentify the functional group(s) in the following molecule. Group of answer choices ether, ketone, amine, cyclic hydrocarbon, aromatic ether, ketone, amide, cyclic hydrocarbon, aromatic ether, ketone, amine, aromatic ether, ketone, amine, cyclic hydrocarbon ether, aldehyde, amine, cyclic hydrocarbon, aromatic ether, ketone, amine, cyclic hydrocarbon, aromatic, carboxylic acidarrow_forward
- Which of the following are reasons carbon is the fundamental building block of organic compounds? Select all that apply. Incorrect selections will deduct credit. O Carbon can form double and triple bonds. O Carbon can bond to many different atoms. O Carbon is very electronegative. O Carbon can break the octet rule. O Carbon can form four bonds.arrow_forwardDraw an example of each of the following under the appropriate name: aldehyde alkyne alcohol epoxide sulfidearrow_forwardFollowing name is incorrect. Write down the correct name using IUPAC system. 3-ethyl-1-butanoic acidarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY