
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
3.Can you help me solve this
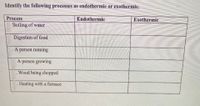
Transcribed Image Text:Identify the following processes as endothermic or exothermic.
Process
Endothermic
Exothermic
Boiling of water
Digestion of food
A person running
A person growing
Wood being chopped
. Heating with a furnace
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many milliliters (mL) of 1.49 M Ba(OH)2 is required to neutralize 11 mL of 6.59 M HBr? O a. 2.38 mL O b. 0.0243 mL O c. 24.3 mL O d. 0.0725 mL O e. 1.90 mLarrow_forwardUse the acidity model pH = −log[H+], where acidity (pH) is a measure of the hydrogen ion concentration [H+] (measured in moles of hydrogen per liter) of a solution.Find the pH when [H+] = 4.7 × 10−5. (Round your answer to two decimal places.)pH =arrow_forwardMatch the following descriptions in column A to the choices listed in column B: A 1. Stoichiometry 2. Spikes 3. Ferromagnetism 4. Colloid 5. Hole 6. Nanoparticle 7. Empirical formula 8. Unit cell 9. Surfactant 10. Ferrofluid 11. Magnetite 12. Van der Waals forces B a. Weak forces of attraction between molecules b. Regions where unpaired electrons strongly interact with one another & align, even in the absence of a magnetic field c. A pattern of uplifted suspended particles that arise from placing a magnet near ferrofluid d. A chemical term that deals with relative amounts of substances involved in chemical reactions e. A dispersion of particles from ~ 1 nm to 1000nm f. A phenomenon in which the internal magnetic moments of unpaired electrons within the domain of the solid are aligned and act cooperatively g. An empty site in a crystalline solid h. A very small particle on a scale of nanometers (10⁹m) i. The name for Fe3O4 j. Information that gives the simplest ratio between atoms of…arrow_forward
- Potassium is a very reactive metal, but in compounds it is present as the potassium ion and is not very reactive. For example, dry potassium bicarbonate powder can be used to extinguish burning liquids. Why is there such a difference in the reactivity of potassium metal and the potassium ion? A. The potassium in the bicarbonate salt is a base, but the potassium metal is an acid. B. The potassium in the bicarbonate salt is an acid, but the potassium metal is a base. C. The combustion of the liquids suppresses the potassium's ability to react. D. The potassium metal can readily ionize by losing its one valence electron; the potassium in the bicarbonate salt is already ionized. E. The potassium atom is bonded to an oxygen atom in the bicarbonate salt, but in the metal it is unbound and free to react.arrow_forwardCompare the following compounds based on the property given.arrow_forwardYour roommate, a history student, has stumbled across nitrogen trifluoride in their history textbook because it was used in the 1960s and 1970s as rocket fuel oxidizer. They remember learning about chemical reactions in Grade 11 and want to make nitrogen trifluoride using nitrogen gas and fluorine gas. Since you are a chemistry student, you have decided to help them remember how to construct the formula. Your other roommate, who is a mathematics student, wants to create a hypothetical situation so that the history student can better understand the concept. She writes down a mass for fluorine gas and nitrogen gas. 1. If she wrote that the mass of nitrogen gas as 114.6 g and the mass of fluorine gas as 354.8 g which is the limiting reagent in this case? 2. The mass of nitrogen trifluoride produced that she wrote is 469.4 g. What is the theoretical mass of nitrogen trifluoride produced calculated using the given masses? Is this higher or lower than the mass that she wrote? 3. If it is…arrow_forward
- To prepare 0.20 M NaOH (40.0 g/mol) you will need to dilute 34 g of NaOH to ____ mL. a.2.6 mL b.4.2 mL c.4200 mL d.170 mL e.590 mLarrow_forwardA 1:20 dilution is done with a 0.2500 M Na,SO, solution. What is the concentration of the diluted solution ? A. 0.5000 M B. 0.0500 M C. 0.1250 M D. 0.01250 Marrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY